Novel synthesis method of CDK4 (cyclin-dependent kinase 4) inhibitor
A synthetic method and inhibitor technology, applied in the field of medicine, can solve problems such as harsh reaction conditions, difficulty in industrial production, and low yield of intermediate preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
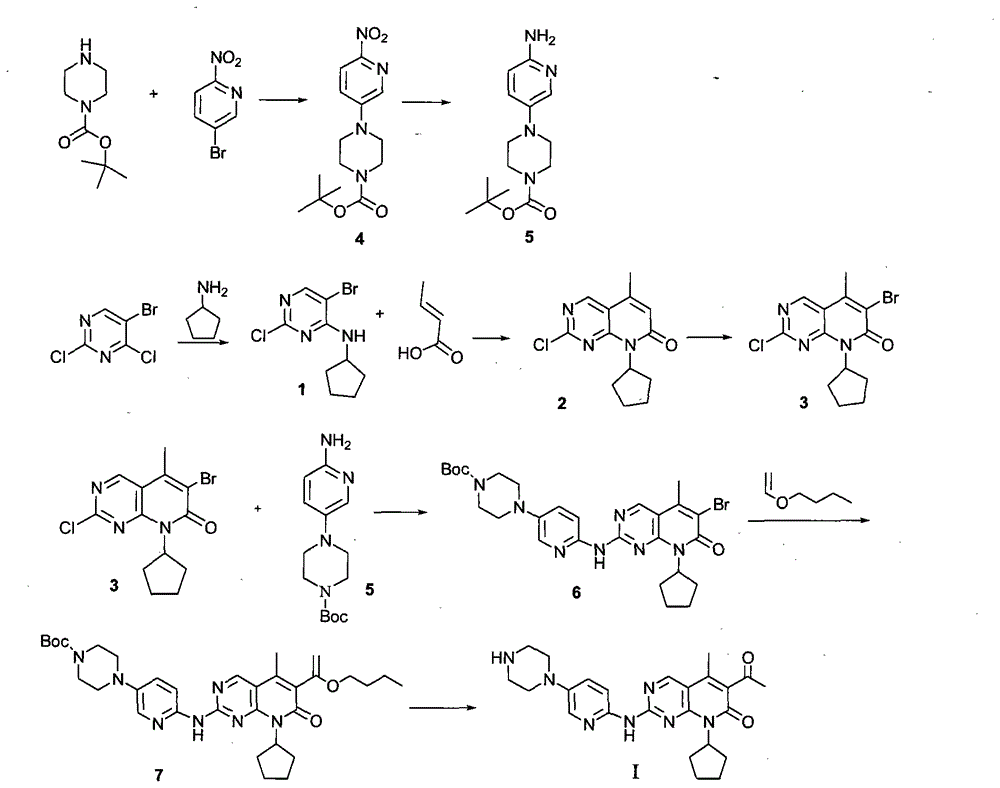
[0062] Embodiment 1: the synthesis of intermediate (1)
[0063] Add 13.2g of 2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one, 6.2g of acetyl chloride, and 65ml of chloroform into the reaction flask, stir to dissolve , the temperature was lowered to 0°C, 10g of anhydrous aluminum chloride was added to the system, the temperature was kept at 10°C for 1 hour, the temperature was raised to 60°C, and the reaction was continued for 4 hours. TLC monitoring (petroleum ether: ethyl acetate = 5: 1) until the reaction at the raw material point was basically complete. Lower the feed solution to room temperature, add 30ml of dilute hydrochloric acid, stir for 5 minutes, let stand, separate the liquid, wash the organic phase with water, wash the organic phase with an appropriate amount of 5% sodium hydroxide solution, wash the organic phase with water, dry over anhydrous sodium sulfate, and filter with suction , concentrated to dryness under reduced pressure at 40°C to obta...
Embodiment 2
[0064] Embodiment 2: the synthesis of intermediate (2)
[0065] Dissolve 10g of the above-mentioned oil intermediate (1) in 100ml of DMF, add 9g of potassium carbonate and 11.8g of tert-butyl 4-(6-amino-pyridin-3-yl)-piperazine-1-carboxylate, stir and heat up To 80°C, keep warm at 85°C and stir for 4 hours. TLC (petroleum ether: ethyl acetate = 1:2) monitors, after the starting point disappears, cool down to room temperature. Pour the feed liquid into water, a large amount of orange-yellow solid is precipitated, filter, wash the filter cake with a large amount of water until the mother liquor is neutral, dry at 50°C, and dry to obtain 14.2g of crude yellow solid, add 300ml of DMSO and heat until dissolved, cool down, and filter with suction. The filter cake was washed with a small amount of ethanol, and dried under reduced pressure at 50°C to obtain 12.5 g of bright yellow intermediate (2). Overall yield: 70%. 1 H NMR (500MHz, DMSO-d 6 ): δ8.15(s, 1H), 7.83(s, 1H), 7.03(d,...
Embodiment 3
[0066] Embodiment 3: the synthesis of compound I
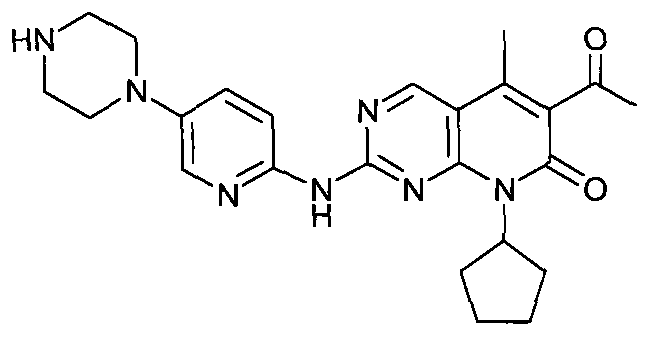
[0067] Add 10 g of intermediate (2) into a mixed solution of 100 ml of acetone and 200 ml of water, stir until the material is uniformly dispersed, add 12 ml of 35% concentrated hydrochloric acid, and heat up to 50° C. for 10 hours. TLC (dichloromethane:methanol, 10:1) monitored until the starting point disappeared. Cool down to 10°C, slowly add 13g of triethylamine to the system until pH > 9, stir well until a large amount of solids are precipitated, continue stirring for 3 hours, filter with suction, wash the filter cake with water until neutral. Dry at 40°C until dry to obtain 7.28g yellow solid I crude product, dissolve the crude product in 150ml n-butanol and 150ml anisole mixture, heat to 100-110°C until dissolved, add 0.5g activated carbon, reflux for 30min and heat filter, The mother liquor was allowed to crystallize naturally at room temperature, filtered, and dried at 40°C to obtain 6.8 g of bright yellow solid comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com