Fluorene spirotriphenylamine derivatives and uses thereof
A compound and device technology, applied in the field of fluorene spirotriphenylamine derivatives, can solve the problems that hinder wide application, low material stability, low glass transition temperature, etc., and achieve lower turn-on voltage, high-efficiency electroluminescent performance, and device The effect of performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Step 1: Dissolve 3.24 g of 2-bromotriphenylamine in 80 mL of tetrahydrofuran under argon protection, cool to -78°C, slowly add 4.38 mL of n-butyllithium into the solution through a constant pressure dropping funnel, and react for 1 hour . Then 1.8 g of fluorenone was dissolved in 40 mL of tetrahydrofuran under the protection of argon and added dropwise to the reaction solution. After 1 hour of reaction at low temperature, it was gradually raised to room temperature. After 12 hours of reaction, 5 mL of water was added to the reaction, and then the solvent was spin-dried under reduced pressure. The solid was dissolved in 80 mL of dichloromethane, and the organic layer was washed three times with 50 mL of water. The organic layer was dried over anhydrous sodium sulfate and spin-dried. The solid obtained by spinning was dissolved in 45 mL of glacial acetic acid and 10 mL of nicotinic acid, refluxed for 4 hours, cooled to room temperature, then suction filtered and washed ...
Embodiment 2
[0047] Step 1: Same as Step 1 of Example 1.
[0048] Step 2: Same as Step 2 of Example 1.
[0049] Step 3: Add 1.11 g of dibromocyclo-closed triphenylamine and 0.68 g of α-N heterocarbazole into a 50 ml flask, add catalyst Pd 2 (dba) 3 92 mg, 80 ml of toluene, 29 mg of tri-tert-butylphosphonium tetrafluoroborate, 30 mg of sodium tert-butoxide, reflux under argon protection for 12 hours, extract with dichloromethane after cooling, dry the organic layer with anhydrous sodium sulfate and spin Dry, pass through the column with dichloromethane / petroleum ether = 1:1 (volume ratio), and spin dry to obtain 1.31 g of SAFNDCZ, with a yield of 88.5%.
Embodiment 3
[0055] The compound SAFNDCZ of the present invention is used as the OLED device host material, FIrpic is a blue phosphorescent dye, and the device structure is:
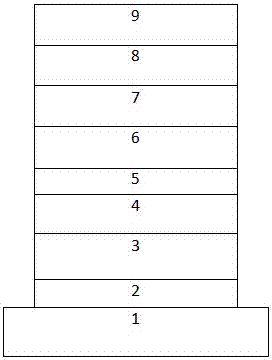
[0056] ITO / HAT-CN(10nm) / TAPC(45nm) / SAFDCZ:FIrpic(20nm, 15vol% doping) / TmPyPB(40nm) / Liq(2nm) / Al(120nm).
[0057] The device preparation process is as follows: the ITO transparent conductive glass substrate is ultrasonically treated in a commercial cleaning agent, rinsed in deionized water, washed repeatedly with deionized water, acetone, and ethanol three times, and baked in a clean environment until the water is completely removed. Treat the ITO conductive glass with UV lamp and ozone. Place the treated ITO conductive glass in a vacuum chamber and evacuate to 3.0×10 -4 -4.0×10 -4 Pa, HAT-CN was vacuum-deposited on ITO conductive glass as a hole injection layer (HIL), the evaporation rate was 0.25Å / s, and the coating thickness was 10nm; TAPC was vacuum-deposited on the hole injection layer as a hole Transport layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com