Optical isomers used as tyrosine kinase inhibitors
An optical isomer and pharmaceutical technology, applied in the field of medicine, can solve problems such as toxic side effects and no efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylthio)pyrimidin-4-ylamino)phenyl)acrylamide preparation of
[0176]
[0177] (1) Preparation of tert-butyl 3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylthio)pyrimidin-4-ylamino)phenylcarbamate
[0178]
[0179] Dissolve tert-butyl 3-(2-chloro-5-(methylthio)pyrimidin-4-ylamino)phenylcarbamate (0.25g, 0.68mmol) in 10mL of tert-amyl alcohol, then add 2 drops of acetic acid , 4-(2-methoxyethoxy)aniline (0.119g, 0.71mmol), the reaction was stirred at 85°C for 1h. The system was spin-dried, and column chromatography (PE:EA=8:1—2:1) gave 0.15 g of a white solid, with a yield of 44.1%.
[0180] (2)N 4 -(3-Aminophenyl)-N 2 Preparation of -(4-(2-methoxyethoxy)phenyl)-5-(methylthio)pyrimidine-2,4-diamine
[0181]
[0182] tert-butyl 3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylthio)pyrimidin-4-ylamino)phenylcarbamate (0.14g, 0.28mmol ) was dissolved in 10 mL of DCM, reacted with HCl gas under ice bath for 1 h, and...
Embodiment 2
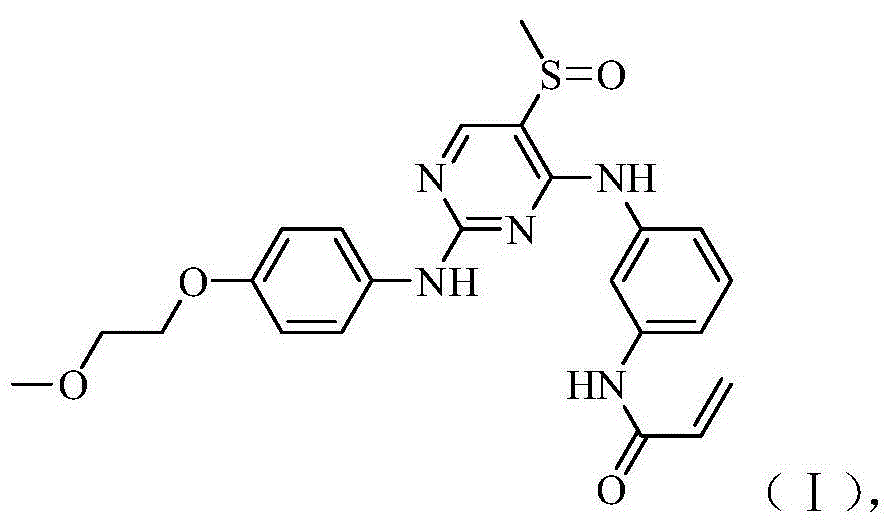
[0188] Example 2N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylsulfinyl)pyrimidin-4-ylamino)phenyl) Preparation of acrylamide
[0189]
[0190] N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylthio)pyrimidin-4-ylamino)phenyl)acrylamide (0.045g, 0.1 mmol) was dissolved in methanol and refluxed for 3 h, spin-dried, and the preparative solution was purified by phase phase (methanol / water=57%) to obtain 9 mg of white solid. Yield: 19.3%.
[0191] Molecular formula: C 23 h 25 N 5 o 4 S Molecular weight: 467.16 Mass spectrum (m / z): 468.2 (M+H) +
[0192] 1 H-NMR (DMSO-d 6 ,400MHz)δ(ppm): 10.17(1H,s),9.54(2H,s),8.20(1H,s),7.73(1H,s),7.55-7.45(3H,m),7.39(1H,d ),7.29(1H,t),6.82-6.73(2H,m),6.42(1H,dd),6.24(1H,dd),5.75(1H,dd),4.00(2H,t),3.62(2H, t), 3.29(3H,s), 2.95(3H,s).
Embodiment 3
[0193] Example 3 (R)-N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylsulfinyl)pyrimidin-4-ylamino)phenyl ) Acrylamide and (S)-N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylsulfinyl)pyrimidin-4-ylamino)phenyl ) C Preparation of enamide
[0194]
[0195] Method 1: Chiral resolution of N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylsulfinyl)pyrimidin-4-ylamino) using chromatographic column ) phenyl) acrylamide, adopt HPLC method, use Daicel's preparation equipment and Daicel chiral column chiral isomer separation, collect its corresponding component, t R =3.694min,t R =4.748min. The solvent was removed by rotary evaporation to obtain the pure product of the optical isomer. HPLC separation conditions:
[0196]
[0197]Method 2: Oxidation of N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylthio)pyrimidin-4-ylamino)phenyl group after addition of chiral reagent ) acrylamide, chiral inducing N-(3-(2-(4-(2-methoxyethoxy)anilino)-5-(methylsulfinyl)pyrimidin-4-ylamino)phenyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com