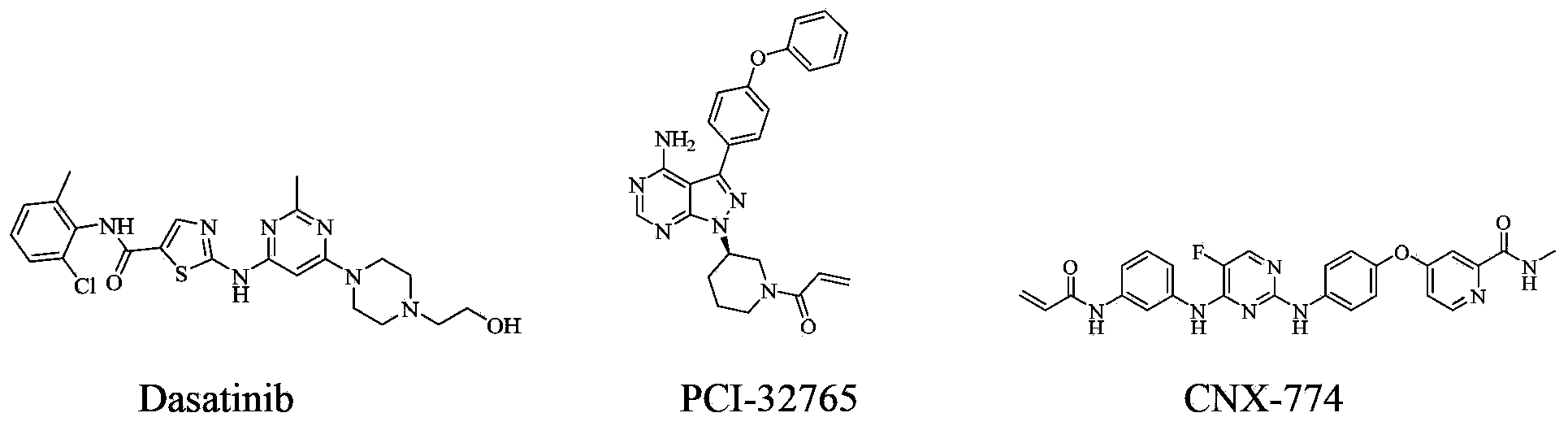
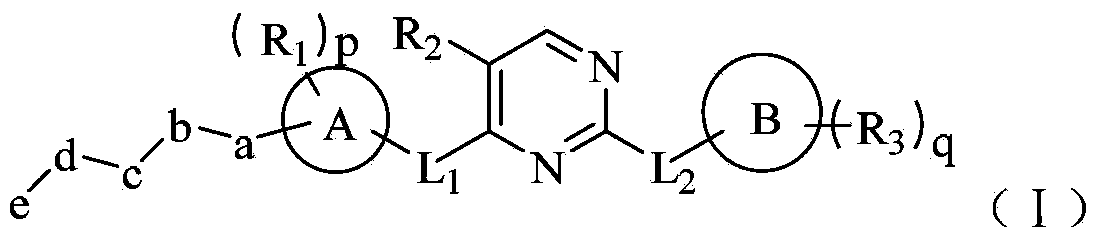
Tyrosine kinase inhibitor for nitrogen hetero-aromatic ring and derivatives thereof
A heteroaryl and amino technology, applied in medical preparations containing active ingredients, anti-inflammatory agents, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0125] The preparation of step 1 intermediate 1
[0126] Mix 5-bromouracil (1 equivalent) with raw material 1 (at least 5 equivalents), without solvent, or add a small amount of polar solvent (water, ethanol, n-butanol, etc.) and heat (100-150 ° C) to react for several hours Until 5-bromouracil disappears. After cooling, a solid precipitated out and was filtered to obtain Intermediate 1. When the raw material 1 is mercaptan, it is necessary to add an equivalent amount of alkali (such as sodium hydroxide) during the reaction.
[0127] Step 2 Preparation of intermediate 2
[0128] Mix Intermediate 1 (1 equivalent) with the solvent amount of phosphorus oxychloride (at least 10 equivalents), add about two equivalents of N,N-dimethylaniline, heat (90-110°C) and stir for several hours to obtain Intermediate 1 disappear. Cooled, poured into ice water, precipitated solid, filtered and dried to obtain Intermediate 2. Or concentrate the reaction system and perform column chromatogr...
Embodiment 1
[0193] Example 14-(4-(4-(3-acrylamidoanilino)-5-aminopyrimidin-2-ylamino)phenoxy)-N-methylpyridine-2-carboxamide (compound 2) preparation
[0194]
[0195] (1) Preparation of tert-butyl 3-(2-chloro-5-nitropyrimidin-4-ylamino)phenylcarbamate
[0196]
[0197] 2,4-Dichloro-5-nitropyrimidine (1.93g, 10mmol) was dissolved in 12mL of THF, cooled to -78°C, tert-butyl 3-aminophenylcarbamate (2.08g , 10mmol) and DIEA (3.3mL, 20mmol) in THF solution 8mL, dropwise, react at this temperature for 2h. Ice water was added to the system, extracted with ethyl acetate, and spin-dried to obtain 3.4 g of a yellow solid with a yield of 93%.
[0198] (2) tert-butyl 3-(2-(4-(2-(methylcarbamoyl)pyridin-4-yloxy)anilino)-5-nitropyrimidin-4-ylamino)phenylcarbamate Preparation of esters
[0199]
[0200] Dissolve tert-butyl 3-(2-chloro-5-nitropyrimidin-4-ylamino)phenylcarbamate (1.83g, 5.0mmol) in 15mL of DMSO, add 4-(4-amino Phenoxy)-N-methylpyridine-2-carboxamide (1.22g, 5.0mmol) and D...
Embodiment 2
[0212] Example 24-(4-(4-(3-acrylamidoanilino)-5-(dimethylamino)pyrimidin-2-ylamino)phenoxy)-N-methylpyridine-2-carboxamide (Compound 3) Preparation
[0213]
[0214] (1) Preparation of 5-(dimethylamino)pyrimidine-2,4(1H,3H)-dione
[0215]
[0216] 5-Bromouracil (10.0g, 52.4mmol) was added to 50mL of 33% dimethylamine aqueous solution, and the reaction was refluxed for 16h after addition. Cool, filter with suction, and dry to obtain 4.0 g of a light yellow solid with a yield of 49%.
[0217] (2) Preparation of 2,4-dichloro-N,N-dimethylpyrimidin-5-amine
[0218]
[0219] Add 5-(dimethylamino)pyrimidine-2,4(1H,3H)-dione (4.0g, 25.8mmol) to 50mL POCl 32mL of N,N-dimethylaniline was added dropwise, and the reaction was carried out at 110°C for 24h after the dropwise completion. Concentrate the reaction system, add 20mL toluene and spin dry the system to obtain an oily substance, pour it into ice water, and wash with Na 2 CO 3 Neutralized to neutral, extracted with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com