Novel oleanolic acid derivatives, and preparation method and application thereof
A technology of oleanolic acid and derivatives, applied in the field of new oleanolic acid derivatives, can solve the problems of low water solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
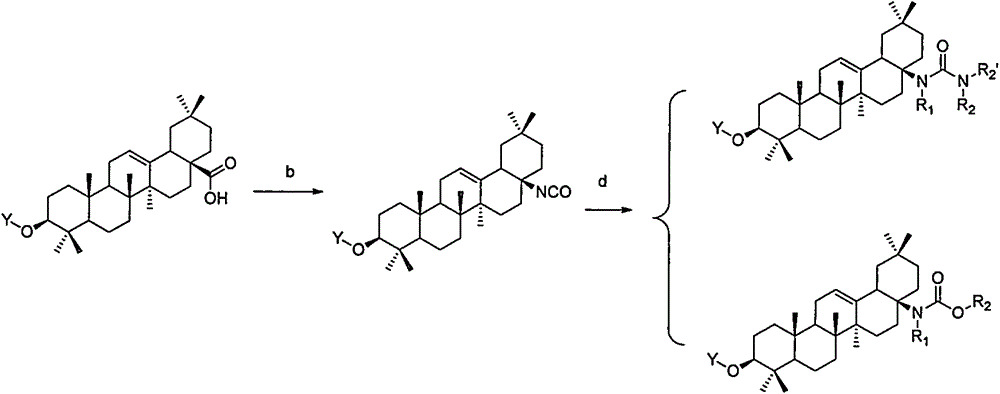
Image
Examples
Embodiment 1
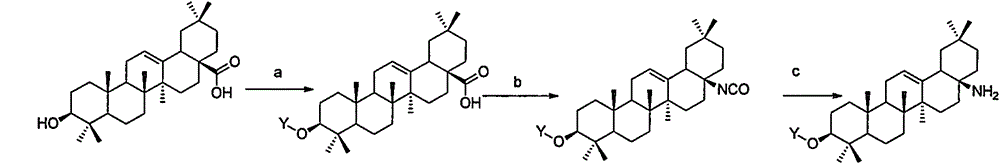
[0061] Embodiment 1: the preparation of compound 1
[0062]
[0063] Suspend 2g of oleanolic acid in 20ml of toluene solution, add 1 equivalent of DPPA (diphenylphosphoryl azide) and 1 equivalent of triethylamine, under nitrogen flow environment, reflux for 3 hours, down to room temperature, the reaction solution with Wash once with 20 ml of saturated potassium carbonate, once with 20 ml of saturated citric acid solution, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 1.5 g of compound 1. LC-Ms: ESI: (M+H) 457.2. 1 H NMR (400MHz, CDCl 3 )δ5.28(t, 1H), 3.24(dd, 1H), 2.39(d, 1H), 2.05(d, 1H), 1.91(m4H), 1.69-1.53(m, 7H), 1.52-1.20(m , 6H), 1.16(s, 3H), 1.08-1.10(m, 1H), 1.04(s, 1H), 1.00(s, 3H), 0.96(d, J=4.4Hz, 1H), 0.93(s, 3H), 0.89(s, 3H), 0.87(s, 3H), 0.83(s, 3H), 0.79(s, 3H), 0.70(d, J=8Hz, 1H).
Embodiment 2
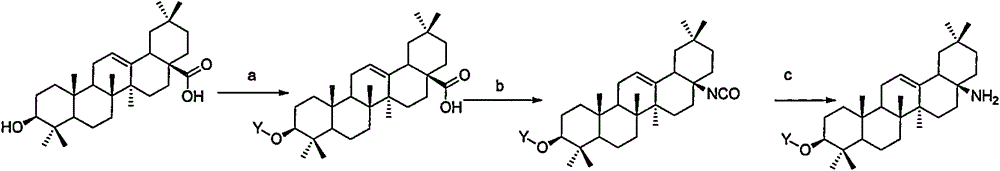
[0064] Embodiment 2: Preparation of OA-1
[0065]
[0066] Dissolve isocyanate in dichloromethane, add 3 equivalents of trifluoroacetic acid, react at room temperature for 3 hours, and concentrate the reaction solution to obtain compound 2 (OA-1). LC-Ms: ESI: (M+H) 428.2. 1 H NMR (400MHz, CDCl 3)δ5.25(t, 1H), 3.28(dd1H), 2.42(d, 1H), 2.10(d, 1H), 1.92(m4H), 1.70-1.54(m, 7H), 1.50-1.20(m, 6H ), 1.18(s, 3H), 1.07-1.09(m, 1H), 1.05(s, 1H), 1.01(s, 3H), 0.96(d1H), 0.93(s, 3H), 0.90(s, 3H) , 0.88(s, 3H), 0.83(s, 3H), 0.81(s, 3H), 0.76(d, J=8Hz, 1H).
Embodiment 3
[0067] Embodiment 3: Preparation of OA-2
[0068]
[0069] Suspend the trifluoroacetic acid salt of compound 2 in dichloromethane, add 2 equivalents of triethylamine dropwise at 0°C, and then add 1.5 equivalents of acetyl chloride dichloromethane solution dropwise at this temperature, and raise the temperature to React at 20°C for 3 hours. The reaction solution was washed with water, dried, concentrated under reduced pressure, and purified by column chromatography to obtain compound 3 (OA-2). LC-Ms: ESI: (M+H) 470.2. 1 H NMR (400MHz, CDCl 3 ( m, 7H), 1.50-1.25(m, 6H), 1.16(s, 3H), 1.06-1.04(m, 1H), 1.03(s, 1H), 1.01(s, 3H), 0.95(d, J= 4.0Hz, 1H), 0.93(s, 3H), 0.89(s, 3H), 0.87(s, 3H), 0.81(s, 3H), 0.77(s, 3H), 0.74(d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com