Substituent acetophenone group purpurine derivative of high-sensitivity color changing material and synthetic method thereof
A technology of acetophenone-based viologen and derivatives, which is applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor stability and low sensitivity of color change, and achieve less side reactions, sensitive response, suitable for The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment one : Synthetic 3,4-dichloroacetophenone substituent viologen, adopt the following steps:
[0028] ① First, take 4,4'-bipyridine (1.56g, 10mmol) and α-chloro-3,4-dichloroacetophenone (5.58g, 25mmol) in a 50 mL single-necked round bottom flask, and use anhydrous DMF (20 mL) dissolved. The solution was refluxed at 120 oC for 24 hours, during which a light gray precipitate was formed.
[0029] ② After the reaction, cool to room temperature, centrifuge to get the precipitate, wash 3 times with anhydrous DMF, 4 times with high-purity water, and 4 times with acetone. The washing solution changes from brownish yellow to almost colorless. After the precipitate was centrifuged and dried in vacuum for 8 hours, the obtained product was 3.83 g (63.47%) of 3,4-dichloroacetophenone substituent viologen.
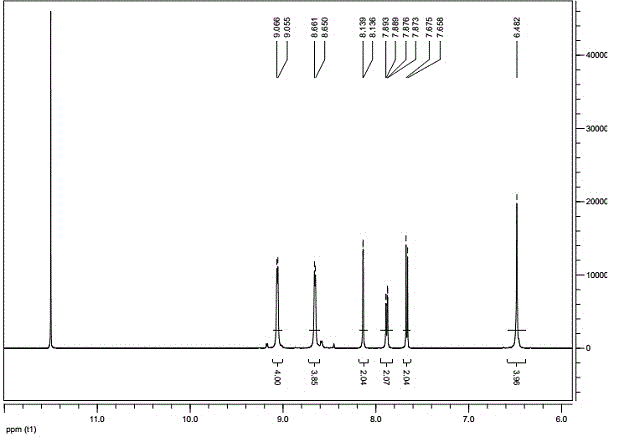
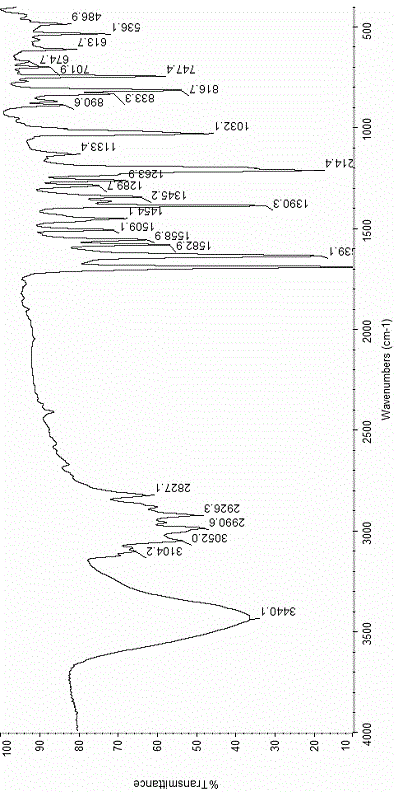
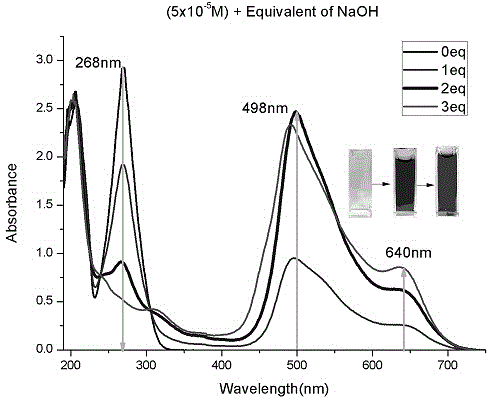
[0030] ③ The physical parameters of the compound: see Figure 1 to Figure 4 .
[0031] ④ Molecular formula: C 26 h 18 N 2 o 2 Cl 6
[0032] ⑤ Structural for...
Embodiment 2
[0041] Embodiment two : Synthesis of 3,4-dihydroxyacetophenone substituent viologen, adopt the following steps:
[0042] ① Weigh 4,4'-bipyridine (1.56g, 10mmol) and 4-chloroacetyl-1,2-catechol (4.66g, 25mmol) in a 50 mL single-necked round bottom flask, and use anhydrous DMF ( 20mL) dissolved. solution in N 2 Stir under protection, heat to 120 oC, and reflux for 24 hours, during which a yellow precipitate is formed.
[0043]② Cool to room temperature after the reaction, centrifuge to get the precipitate, wash it with anhydrous DMF and acetone for 3~5 times respectively, centrifuge and vacuum dry for 8 hours, the product obtained is 3,4-dihydroxyacetophenone substituent viologen 2.25g (64.5%).
[0044] ③ The physical parameters of the compound: see Figure 5 to Figure 8
[0045] ④ Molecular formula: C 26 h 22 o 6 N 2 Cl 2
[0046] ⑤ Structural formula:
[0047]
[0048] ⑥ Chinese name: 3,4-dihydroxyacetophenone instead of viologen
[0049] ⑦ English name: 3,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com