Method for measuring danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid in human blood plasma
A technique for meta-methyl salvia and protocatechualdehyde, which is used in measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 1. Preparation of internal standard solution
[0098] Precisely measure 10.0mg of vanillic acid (internal standard) and put it in a 50mL volumetric flask, dissolve it with methanol and dilute to the mark, and shake well to obtain a 200μg / mL internal standard stock solution. Precisely measure 125μL of this internal standard stock solution in In a 5mL volumetric flask, dissolve with methanol and dilute to the mark to obtain a 5μg / mL internal standard solution.
[0099] 2. Plasma sample processing method
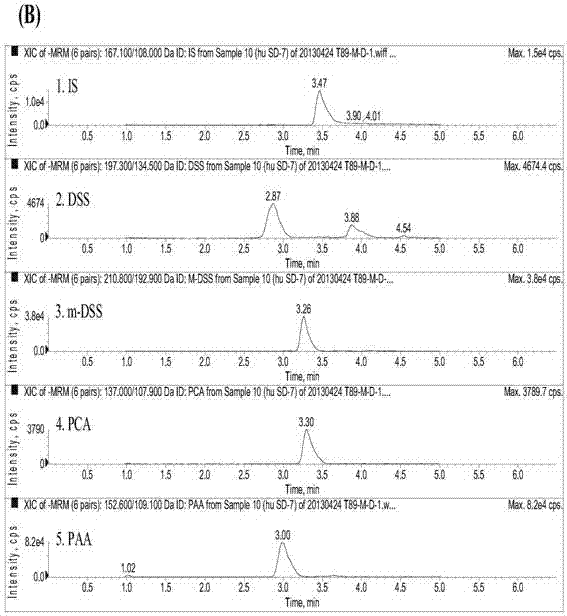
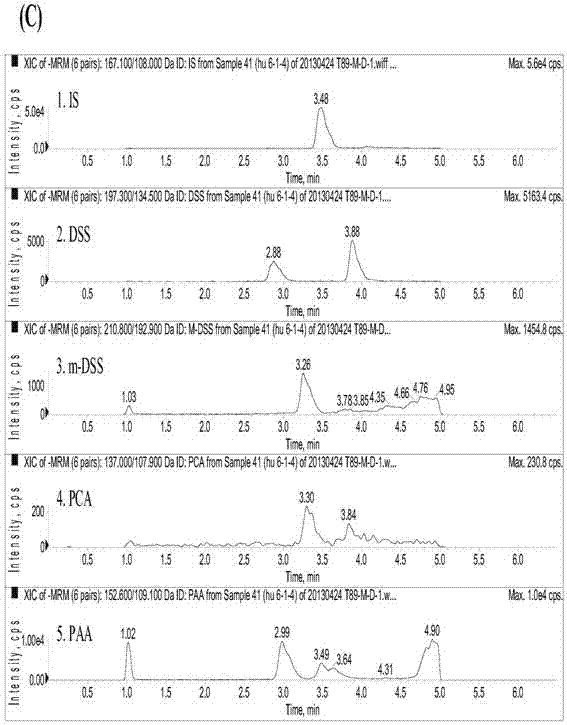
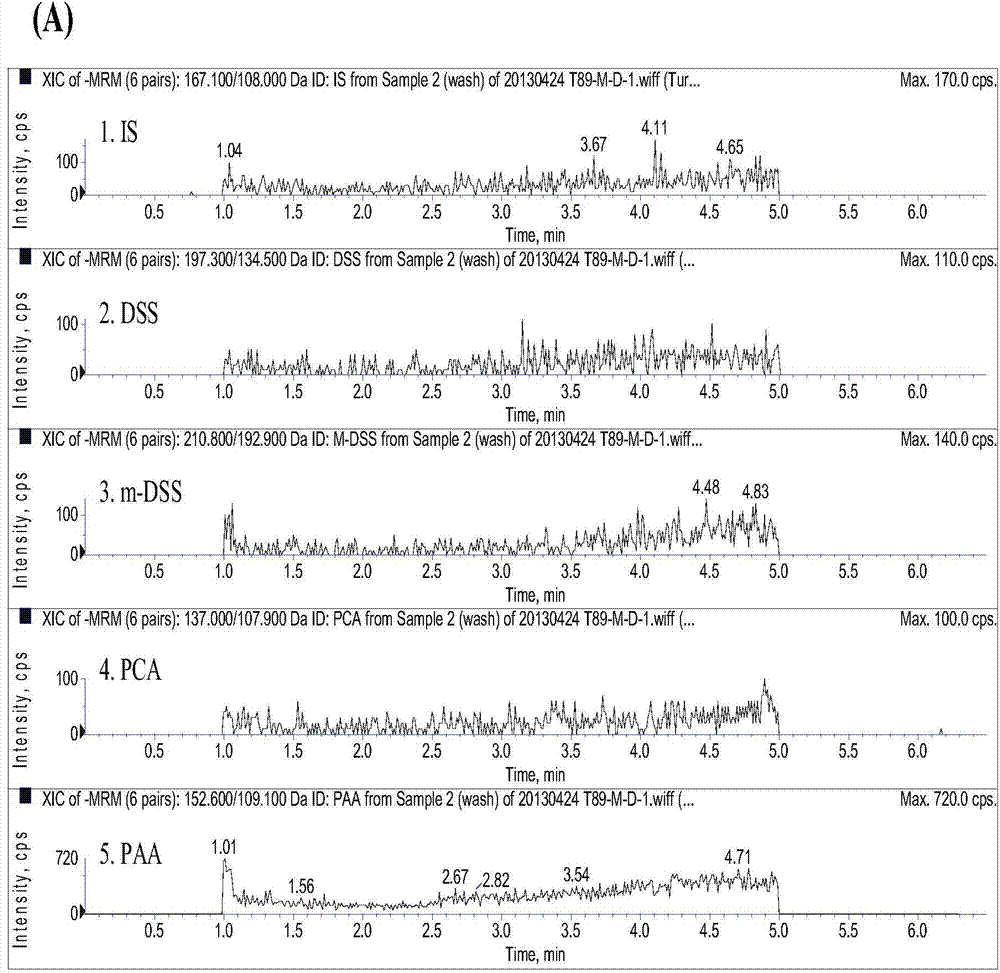
[0100] Take 200 μL of plasma sample, add 50 μL of internal standard solution, 50 μL of 50% methanol aqueous solution, 20 μL of 1mol / L hydrochloric acid solution, vortex evenly, add 1 mL of ethyl acetate, vortex for 30 s, centrifuge at 15000 rpm for 1 min, and take the supernatant ethyl acetate 900 μL of the ester layer was dried under nitrogen flow at 40°C, reconstituted with 120 μL of 50% aqueous methanol solution, centrifuged at 15,000 rpm for 3 min, and 10 μL of the ...
Embodiment 2
[0106] 1. Preparation of internal standard solution
[0107] Precisely measure 10.0mg of vanillic acid (internal standard) and put it in a 50mL volumetric flask, dissolve it with methanol and dilute to the mark, and shake well to obtain a 200μg / mL internal standard stock solution. Precisely measure 125μL of this internal standard stock solution in In a 5mL volumetric flask, dissolve with methanol and dilute to the mark to obtain a 5μg / mL internal standard solution.
[0108] 2. Plasma sample processing method
[0109] Take 200 μL of plasma sample, add 50 μL of internal standard solution, 50 μL of 50% methanol aqueous solution, 20 μL of 1mol / L hydrochloric acid solution, vortex evenly, add 1 mL of ethyl acetate, vortex for 30 s, centrifuge at 15000 rpm for 1 min, and take the supernatant ethyl acetate 900 μL of the ester layer was dried under nitrogen flow at 40°C, reconstituted with 120 μL of 50% aqueous methanol solution, centrifuged at 15,000 rpm for 3 min, and 10 μL of the ...
Embodiment 3
[0114] 1. Preparation of internal standard solution
[0115] Precisely measure 10.0mg of vanillic acid (internal standard) and put it in a 50mL volumetric flask, dissolve it with methanol and dilute to the mark, and shake well to obtain a 200μg / mL internal standard stock solution. Precisely measure 125μL of this internal standard stock solution in In a 5mL volumetric flask, dissolve with methanol and dilute to the mark to obtain a 5μg / mL internal standard solution.
[0116] 2. Plasma sample processing method
[0117] Take 200 μL of plasma sample, add 50 μL of internal standard solution, 50 μL of 50% methanol aqueous solution, 20 μL of 1mol / L hydrochloric acid solution, vortex evenly, add 1 mL of ethyl acetate, vortex for 30 s, centrifuge at 15000 rpm for 1 min, and take the supernatant ethyl acetate 900 μL of the ester layer was dried under nitrogen flow at 40°C, reconstituted with 120 μL of 50% aqueous methanol solution, centrifuged at 15,000 rpm for 3 min, and 10 μL of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com