Application of Lambda-carrageenan to preparation of immunologic adjuvant and antigen-adjuvant compound
A technology of immune adjuvant and carrageenan, which is applied in the field of biomedicine, can solve the problems of preparing vaccines, and achieve the effect of high clinical safety, simple method and strong immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
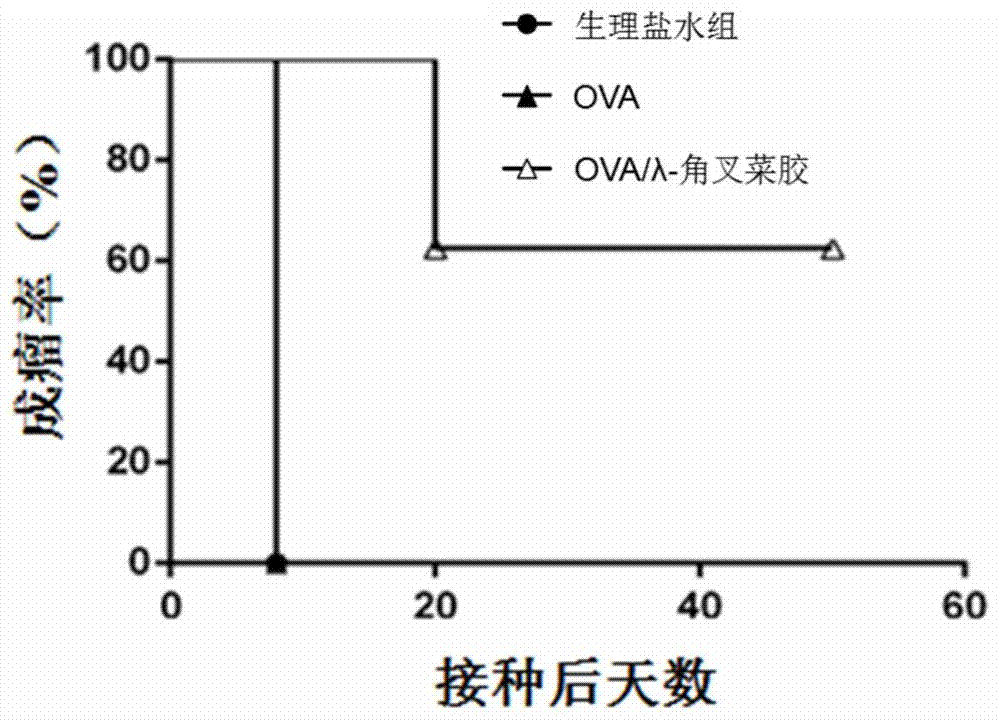
[0040] Example 1 OVA vaccine prepared using λ-carrageenan as an adjuvant and its immunotherapy experiment
[0041] Experimental materials and reagents: λ-carrageenan (CAT.22049) and OVA (CAT.A5503) were purchased from Sigma Company in the United States; C57BL / 6 mice were purchased from Beijing Weitong Lihua Company; mouse lymphoma cells EG .7-OVA was purchased from ATCC in the United States; ELISA self-coated plates were purchased from NUNC.
[0042] (1) Preparation of OVA vaccine
[0043] Preparation of OVA solution: Weigh 10 mg of OVA and dissolve it in 10 mL of normal saline to make a solution with a concentration of 1 mg / mL; then dilute 10 times with normal saline to obtain a stock solution of 0.1 mg / mL.
[0044] Preparation of λ-carrageenan solution: 10 mg λ-carrageenan was weighed and dissolved in 5 mL of normal saline to prepare a stock solution with a concentration of 2 mg / mL.
[0045] Take 50 μl of the prepared OVA and λ-carrageenan solutions and mix them in equal v...
Embodiment 2
[0053] Example 2 Discussion on the Immunological Activation of λ-Carrageenan
[0054] Experimental materials and reagents: λ-carrageenan was purchased from Sigma Company (CAT.22049) in the United States; C57BL / 6 mice were purchased from Beijing Weitong Lihua Company; mouse melanoma B16-F10 was purchased from ATCC in the United States; Antibodies for flow cytometry were purchased from BD-Pharmingen, USA.
[0055] (1) Effects of λ-carrageenan treatment on immune function in mice
[0056] B16-F10 tumors were placed subcutaneously on the right side of the mouse, 10 6 / mouse, when the tumor was palpable (about 3mm, 3-5 days after inoculation), the mice were randomly divided into two groups, and the mice began to be injected with normal saline NS and λ-carrageenan intratumorally, 50mg / kg body weight, once a day.
[0057] At the later stage of λ-carrageenan treatment, 3 mice in each group were sacrificed, the tumor tissue was cut into pieces and digested with collagenase to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com