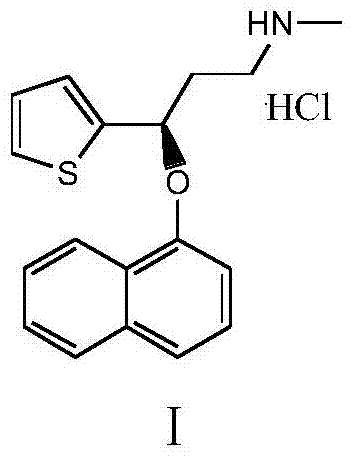
Preparation of duloxetine hydrochloride
A technology of duloxetine hydrochloride and mandelic acid, which is applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of increasing production cycle, restricting industrial production, and increasing costs, and achieves the goals of reducing salt-forming steps, shortening production cycle, and saving costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
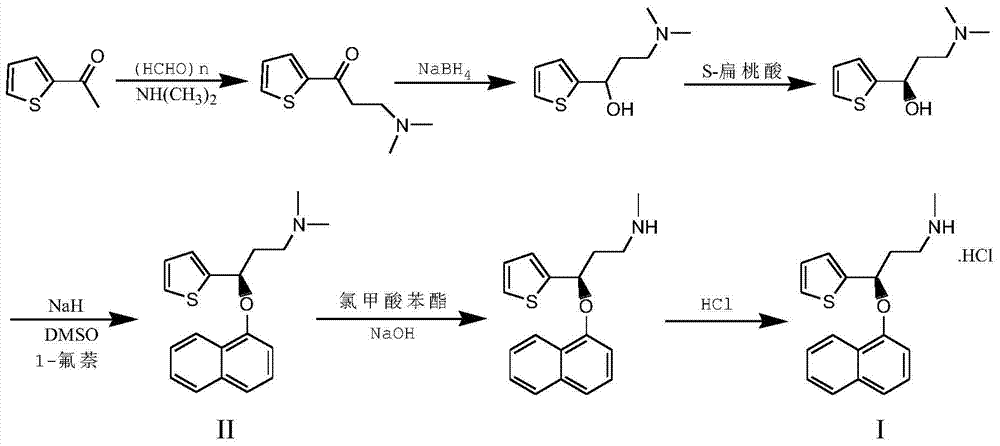
Embodiment 1
[0054] A: Preparation of 2-thiophene-2-dimethylaminomethyl ethyl ketone hydrochloride
[0055] Dissolve 55g (0.44mol) 2-acetylthiophene, 44.7g (0.55mol) dimethylamine hydrochloride, 19.4g (0.61mol) paraformaldehyde in 190ml isopropanol, add 4.3ml concentrated hydrochloric acid and reflux for reaction After 8 hours, it was cooled to room temperature and filtered with suction. The filter cake was washed with 50 ml of cold ethanol and dried to obtain 88.7 g of white solid with a yield of 92.4%.
[0056] B: Preparation of N,N-dimethyl-3hydroxy-3-(2-thiophene)-propylamine
[0057] 85g (0.38mol) 2-thiophene-2-dimethylaminomethyl ethyl ketone hydrochloride, 260ml ethanol and 130ml water are mixed and stirred to dissolve, slowly add 13.9g NaOH at room temperature, adjust the PH value to 11-12, then add 14.5 g (0.37 mol) of sodium borohydride was reacted at room temperature overnight. After the reaction was completed, 160 ml of acetone was added to quench the reaction, and the ethanol was ev...
Embodiment 2
[0067] A: Preparation of (S)-N,N-dimethyl-3-p-nitrobenzoate-3-(2-thienyl)propylamine
[0068] 29g (0.16mol) (R)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propylamine (prepared by resolution in Example 1), 31g (0.19mol) p-nitrobenzene Formic acid, 49.4g (0.19mol) triphenylphosphorus, mixed and dissolved in 240ml tetrahydrofuran, cooled to 0~5℃ in an ice bath, slowly dripped 32.9g (0.19mol) diethyl azodicarboxylate, the temperature did not exceed At 10°C, after dropping the ice bath, warming to room temperature, and reacting overnight. After the reaction is completed by TLC, 150ml of dichloromethane is added to the reaction solution, washed with saturated sodium carbonate solution, the aqueous phase is extracted with 80ml of dichloromethane, and the organic phases are combined , Dried with anhydrous magnesium sulfate, filtered, evaporated to dryness under reduced pressure to obtain a reddish brown oil, which was directly used in the next step without treatment.
[0069] B: Preparation of ...
Embodiment 1,2
[0079] Comparison of main reaction conditions and yields of Examples 1, 2 and Comparative Examples
[0080]
[0081] It can be seen from the comparison of the above embodiments:
[0082] 1. After the R-configuration compound obtained by the resolution is converted into the S-configuration, the total yield of duloxetine hydrochloride prepared by the S-configuration alone is increased by nearly 47% (increased yield=[ Total yield of R-configuration preparation / (total yield of R-configuration preparation+total yield of S-configuration preparation)]×100%).
[0083] 2. Using 1-chloroethyl chloroformate instead of phenyl chloroformate during demethylation not only greatly shortens the reaction time, but also directly generates duloxetine hydrochloride, which reduces the reaction steps and shortens the production cycle ( It is shortened by about 44h), and the yield is also increased by nearly 7%, which reduces the production cost.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com