Double-triazole substituted ethoxy benzene compound and preparation method and application thereof
A technology of diethoxybenzene and diethoxyphenyl, which is applied in the field of synthesis of ethoxybenzene compounds, can solve problems such as unretrieved and undetected, and achieve high reaction yield, high purity, high The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of 1,4-bis(bromomethyl)-2,5-diethoxybenzene (compound I) is as follows:
[0034] (1) Add p-bromophenol (1.10 g, 10 mmol), ethyl bromide (2.26 mL, 30 mmol), sodium hydroxide (1.10 g, 10 mmol), 20 mL of methanol, and reacted at 60°C for three hours. After the reaction, the reaction system was lowered to room temperature, poured into water, and a large amount of precipitate was precipitated. After suction filtration, the filter cake was collected and recrystallized with 95% ethanol to obtain 1 , 4-diethoxybenzene, yield 91%; melting point 71-72 ° C.
[0035](2) Add 1,4-diethoxybenzene (1.66 g, 10 mmol), paraformaldehyde (0.75 g , 25 mmol), potassium bromide (2.98 g, 25 mmol), 20 mL of glacial acetic acid, 5 mL of concentrated sulfuric acid was slowly added dropwise through a constant pressure dropping funnel, and reacted at 60°C for six hours. After the reaction, the reaction system was Cool down to room temperature, pour into water, precipitate a ...
Embodiment 2
[0037] The preparation method of 3-(1H-1,2,4-triazol-1-yl)propionitrile (compound II) is as follows:
[0038] Add 1H-1,2,4-triazole (1.38 g, 20 mmol) and 20 mL of toluene to a 50 mL single-necked flask equipped with a magnet, reflux condenser and constant pressure dropping funnel, and add acrylonitrile dropwise (1.31mL, 20mmol), refluxed for 20 hours, after the reaction, the reaction system was lowered to room temperature, and the toluene was removed to obtain 3-(1H-1,2,4-triazol-1-yl)propionitrile. The rate is 89%. The melting point is 35-36°C.
Embodiment 3
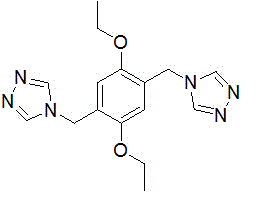
[0040] 4-(4-((4H-1,2,4-triazol-4-yl)methyl)2,5-diethoxyphenyl)-4H-1,2,4-triazole The preparation method is as follows:
[0041] Add 1,4-bis(bromomethyl)-2,5-diethoxybenzene (3.48 g, 10 mmol) into a 100 mL single-necked flask equipped with a magnet, reflux condenser and constant pressure dropping funnel, 3-(1H-1,2,4-triazol-1-yl)propionitrile (2.44 g, 20 mmol), 60 mL of acetonitrile, refluxed for 6 hours, distilled off the acetonitrile, added 1mol / L hydroxide Sodium aqueous solution 30mL, stirred at room temperature for 24 hours, extracted with dichloromethane, and the organic layer was washed with NaSO 4 Dry overnight, filter magnesium sulfate, and distill off dichloromethane to obtain 4-(4-((4H-1,2,4-triazol-4-yl)methyl)2,5-diethoxybenzene Base)-4H-1,2,4-triazole, yield 81%. Melting point 244-245°C;
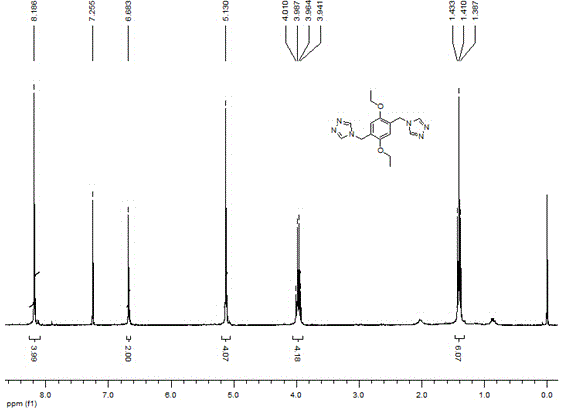
[0042] 1 NMR (400 MHz, CDCl 3 ) : δ 8.19 (s, 4H), 6.68 (s, 2H), 5.13 (s, 4H), 4.01-3.94 (q, 4H), 1.43-1.39 (t, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com