Storage stabilizer for human serum immunoglobulin solution preparations
A storage stabilizer and immunoglobulin technology, which is applied in the direction of medical preparations and pharmaceutical formulas containing active ingredients, and can solve the problem of high degree of polymerization of immunoglobulins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
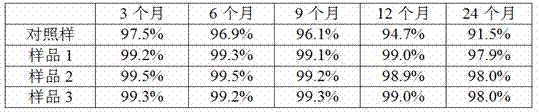
[0011] Take human serum immunoglobulin chromatography solution (purity 99.2%, concentration 0.37mmol / L) after virus inactivation, concentrate by ultrafiltration to prepare immunoglobulin solution with a concentration of 10wt%, take 1L sample, add glucose to 230mmol / L L, adjust the pH to 4.1 as a blank control sample, take another 3 L, add glutathione to 6mmol / L, maltose to 300mmol / L, proline to 50mmol / L, adjust the pH to 4.1, divide into sample 1 and sample 2 , Sample 3 each 1L. The molecular size distribution of the product was investigated under storage conditions at room temperature.
[0012] The molecular size distribution is checked by high-performance liquid chromatography: a hydrophilic silica gel high-efficiency size-exclusion chromatography column (SEC, exclusion limit 300kD, particle size 10μm), column diameter 7.5mm, length 60cm; with 1% isopropanol, pH value 7.0, 0.2mol / L phosphate buffer (take 200ml of 0.5mol / L sodium dihydrogen phosphate, 420ml of 0.5mol / L disod...
Embodiment 2
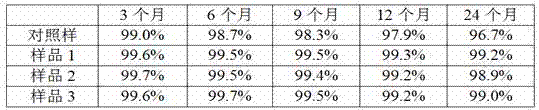
[0016] Take human serum immunoglobulin chromatographic solution (purity 99.5%, concentration 0.25mmol / L) after virus inactivation, concentrate by ultrafiltration to prepare an immunoglobulin solution with a concentration of 10wt%, take 1L sample, add glucose to 220mmol / L L, adjust the pH to 3.85 as a blank control sample, take another 3 L, add cysteine to 4mmol / L, maltose to 500mmol / L, glycine to 130mmol / L, adjust the pH to 3.85, respectively for sample 1, sample 2, Sample 3. The molecular size distribution of the product was investigated under the storage condition of 37°C.
[0017] The molecular size distribution was checked by high performance liquid chromatography according to Example 1. The immunoglobulin monomer plus dimer content was measured at 3, 6, 9, 12 and 24 months of storage, and the results are shown in the table below.
[0018]
Embodiment 3
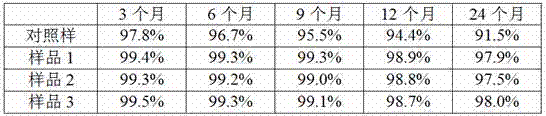
[0020] Take the immunoglobulin chromatography solution after virus inactivation (purity 98.7%, concentration 0.27mmol / L), concentrate by ultrafiltration to prepare an immunoglobulin solution with a concentration of 5wt%, take 1L sample, add glucose to 225mmol / L, Adjust pH to 6.9 as blank control sample, take another 3L, add glutathione to 8mmol / L, maltose to 250mmol / L, serine to 75mmol / L, adjust pH to 6.9, divide into sample 1, sample 2, sample 3 1L. The molecular size distribution of the product was investigated under the storage condition of 37°C.
[0021] The molecular size distribution was checked by high performance liquid chromatography according to Example 1. The immunoglobulin monomer plus dimer content was measured at 3, 6, 9, 12 and 24 months of storage, and the results are shown in the table below.
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com