Application of Chinese medicine monomer (lycorine) to preparation of drugs for treating prostate tumors
A technology of lycorine and prostate cancer, applied in the field of medicine, can solve the problems of unsatisfactory curative effect of prostate cancer, recurrence of multidrug-resistant tumors, poor targeting of anti-prostate cancer drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
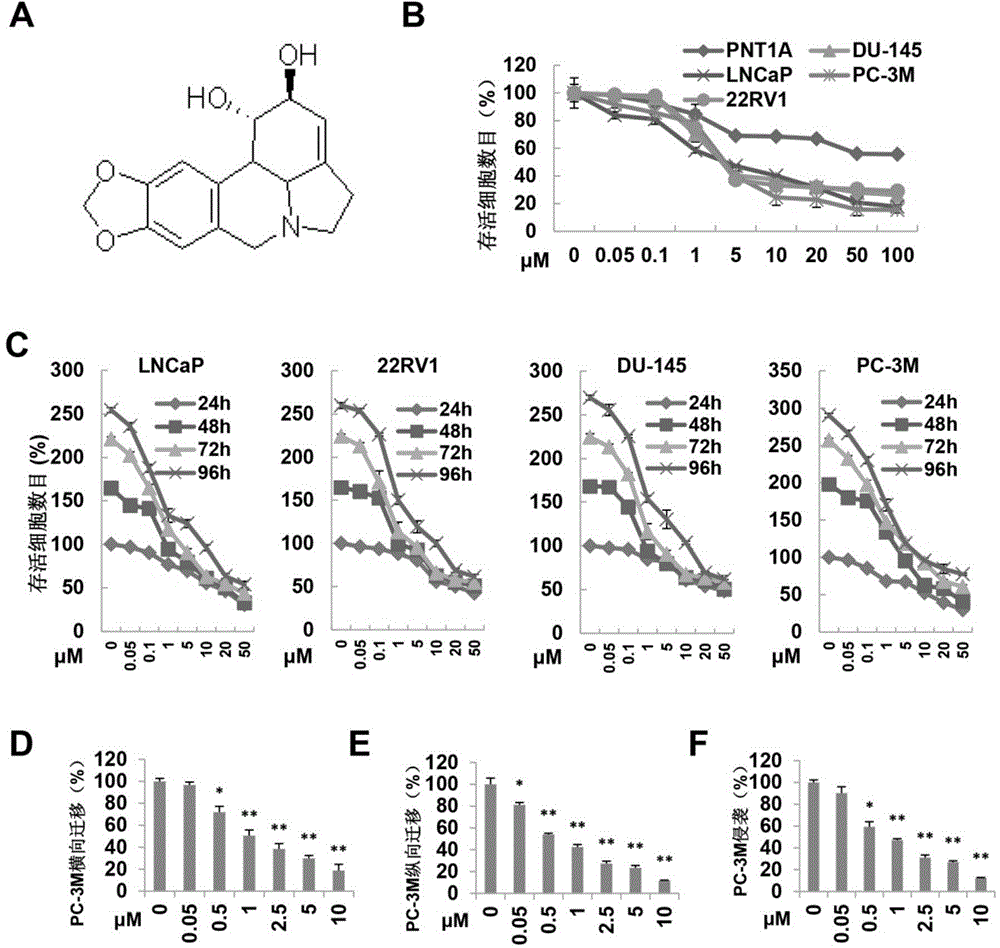
[0026] Example 1: Lycorine inhibits the proliferation, migration and invasion of prostate cancer cells
[0027] Technical method
[0028] 1. Cell culture and cell survival experiments
[0029] Prostate cancer cells PC-3M, LNCaP, 22RV1 and DU145 used in the present invention were purchased from ATCC cell bank, and human normal prostate epithelial cell line PNT1A was obtained from Shanghai Jiaotong University. The cells were cultured in a constant temperature incubator at 37°C (humidity 95%, CO 2 Concentration 5%), the medium is RPMI1640 containing 10% fetal bovine serum. Cell viability was determined by MTS method. Prostate cell lines PC-3M, LNCaP, 22RV1 and DU145, and human normal prostate epithelial cell line PNT1A in 5x10 3 Cells / well density was inoculated into 96-well plates, and different concentrations of the monomer compound were added after 24 hours, and the same amount of DMSO was added to the control group, and 3 replicate wells were set up in each group. After ...
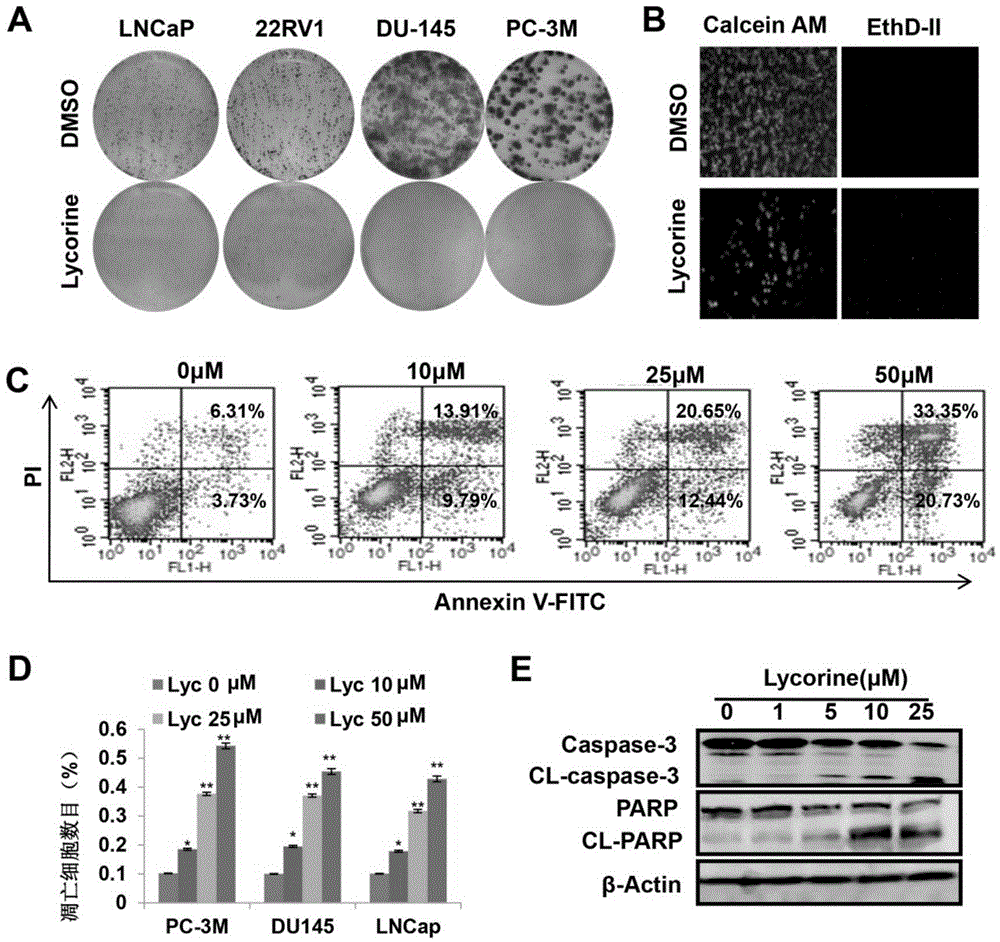
Embodiment 2
[0036] Example 2: Lycorine inhibits the growth of prostate cancer cells in vitro by inducing apoptosis
[0037] Technical method
[0038] 1. Colony formation experiment
[0039] Digest and process the corresponding cells in the logarithmic growth phase, and count, 1x10 per well 3 Cells were seeded in a six-well plate to ensure that the seeded cells were evenly distributed. After the cells adhere to the wall, change the medium and add the complete medium containing the corresponding concentration of the monomer compound. After culturing for one week, suck up the original culture medium with a suction pump, wash 3 times with phosphate buffer solution, fix the cells with paraformaldehyde solution (20 min), then stain the cells with 2‰ crystal violet staining solution for 5 min, and finally use Rinse gently with slow-flowing tap water to wash away unbound crystal violet staining solution, and dry naturally at room temperature. Photographs were taken under a microscope, and the...
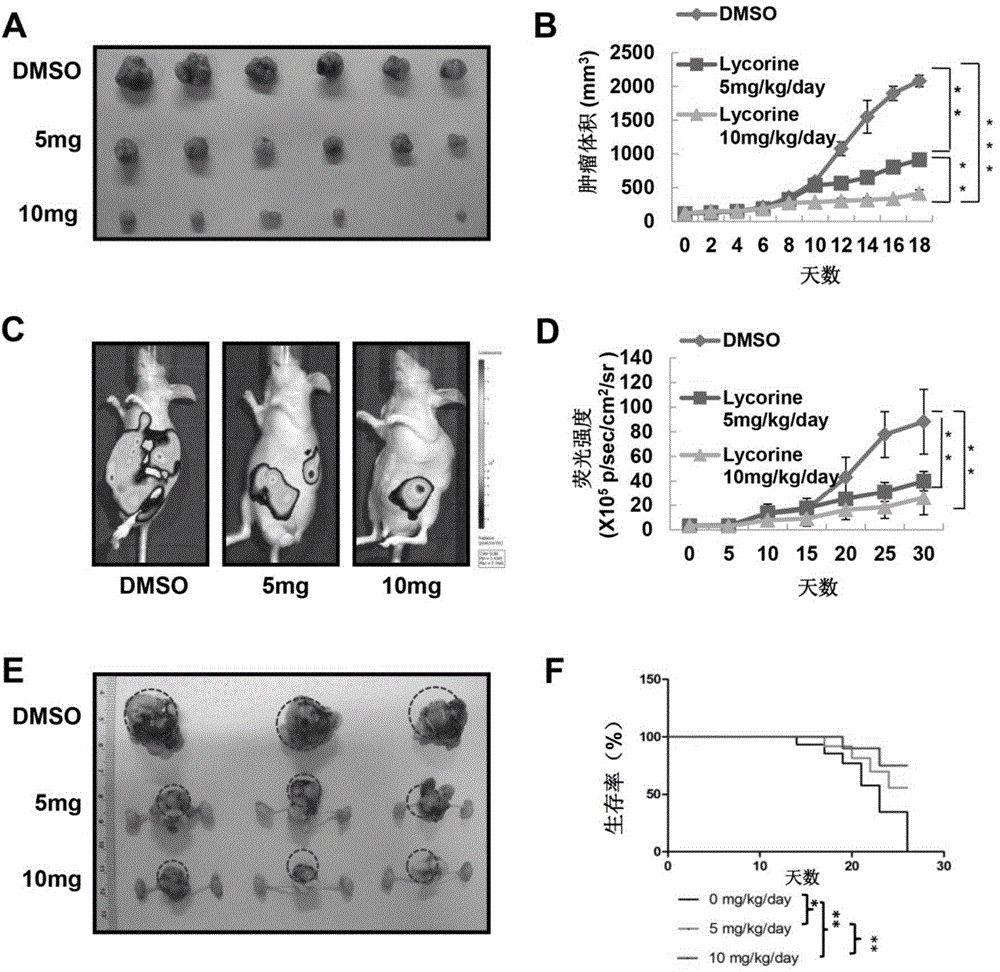
Embodiment 3
[0048] Example 3: Inhibitory effect of lycorine on subcutaneous tumor-bearing growth of mouse prostate cancer model
[0049] Technical method
[0050] Will 1×10 6 Personal prostate cancer cell PC-3M was subcutaneously injected into the back of immunodeficient mice (BLAB / c-nude, nude mice) until the subcutaneous tumor grew to 100mm 3 When left and right, the mice were divided into three groups (10 mice in each group). The mice in the low-dose group were injected intraperitoneally with 5 mg / kg lycorine dissolved in DMSO every day, the mice in the high-dose group were injected intraperitoneally with 10 mg / kg lycorine dissolved in DMSO every day, and the mice in the control group were injected with the same volume of DMSO. The body weight of the mice and the length and width of the tumor were recorded, and the mice were sacrificed after 18 days of continuous drug administration. Subcutaneous tumors were removed and photographed. According to the formula volume = length x width...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com