Pyran ring-containing negative liquid crystal compound and preparation method thereof
A technology for negative liquid crystals and compounds, applied in the field of negative liquid crystal compounds and their preparation, can solve the problems of poor mutual solubility, high melting point and viscosity, and lack of obvious improvement in the optical anisotropy and dielectric anisotropy of liquid crystal mixtures. , to achieve the effect of improving low temperature performance, good low temperature stability, and improving light response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0045] 1. Synthesis of compound b-1
[0046]Under nitrogen protection at a flow rate of 0.6mL / min, 10.00g of 4-bromo-2,3-difluorophenol (compound a-1), 6.05g of 3,4-2H-dihydropyran, 1.21g of 4- Pyridinium toluenesulfonate (PPTS, CAS number: 24057-28-1, provided by Chengdu Best Reagent Co., Ltd.) and 100 mL of anhydrous dichloromethane were added to a three-necked flask equipped with a thermometer, a magnetic stir bar, and a condenser , stirred and reacted at room temperature for 4 hours, the resulting reaction solution was diluted with saturated aqueous sodium bicarbonate solution, the organic layer was separated, the aqueous layer was extracted three times with dichloromethane, the organic phases were combined and washed with water until neutral, and dried with anhydrous magnesium sulfate Concentrate to obtain an oily liquid, and the obtained concentrated solution is subjected to column chromatography separation and purification (using silica gel as a stationary phase, and a ...
Embodiment 2
[0058] 1. Synthesis of compound b-1
[0059] The specific synthesis method is the same as Step 1 of Example 1.
[0060] 2. Synthesis of negative liquid crystal compounds containing pyran rings
[0061] In step 2 of Example 1, the 2-methyl-4-(4-trans-(4-n-propylcyclohexyl) ethylphenyl)-3-butyn-2-ol (compound c- 1) Replace with equimolar 2-methyl-4-(4-trans-(4-n-ethylcyclohexyl)ethylphenyl)-3-butyn-2-ol (compound c-2), other The steps are the same as in Example 1 to obtain white crystals—a negative liquid crystal compound containing a pyran ring, whose chemical name is 1-{4-[2-(4-n-ethylcyclohexyl)ethyl]phenyl}- 2-{4-[(2-tetrahydropyran)oxy]-2,3-difluorophenyl}acetylene, the yield is 45%, the specific reaction equation is as follows:
[0062]
[0063] The structural characterization data of the obtained negative liquid crystal compound containing pyran ring are as follows:
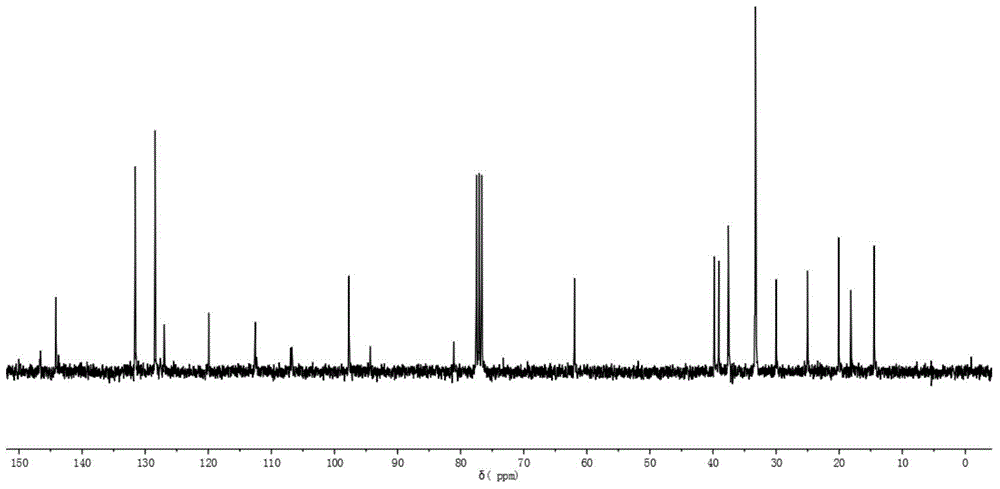
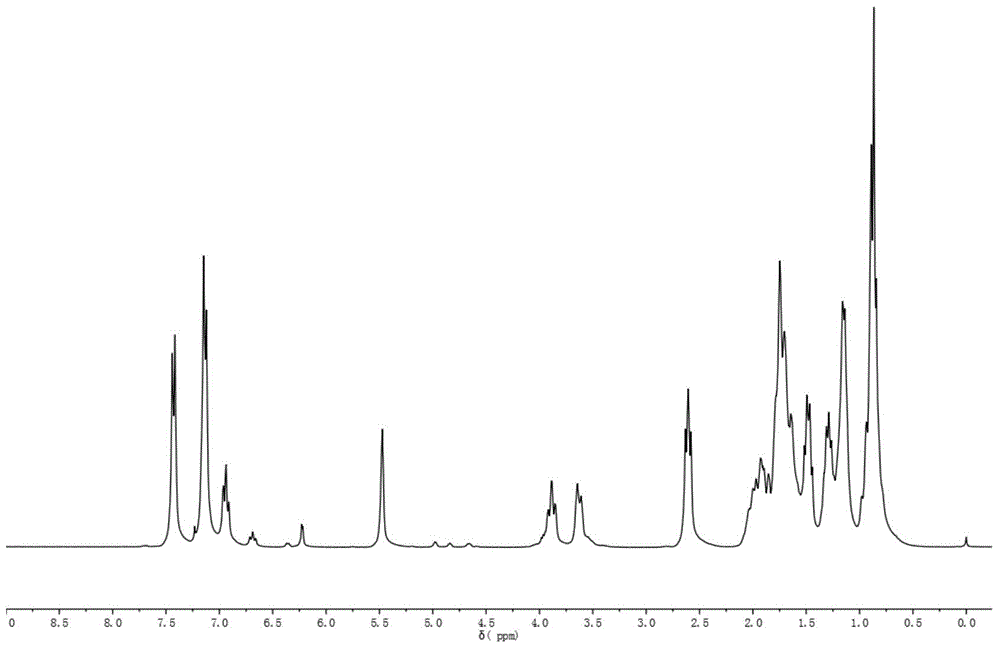
[0064] 13 C-NMR (CDCl 3 is the solvent, the internal standard is TMS, 75MHz, ppm): 146.0, 144.1,...
Embodiment 3
[0070] 1. Synthesis of compound b-1
[0071] The specific synthesis method is the same as Step 1 of Example 1.
[0072] 2. Synthesis of negative liquid crystal compounds containing pyran rings
[0073] In step 2 of Example 1, the 2-methyl-4-(4-trans-(4-n-propylcyclohexyl) ethylphenyl)-3-butyn-2-ol (compound c- 1) Replace with equimolar 2-methyl-4-(4-trans-(4-n-butylcyclohexyl) ethylphenyl)-3-butyn-2-alcohol (compound c-3), other steps and Same as Example 1, white crystals are obtained—a negative liquid crystal compound containing a pyran ring, and its chemical designation is 1-{4-[2-(4-n-butylcyclohexyl)ethyl]phenyl}-2-{4 -[(2-tetrahydropyran)oxy]-2,3-difluorophenyl}acetylene, the yield is 40%, and the specific reaction equation is as follows:
[0074]
[0075] The obtained negative liquid crystal compound containing a pyran ring was characterized by NMR and mass spectrometry, which indicated that it was the target product, and its optical anisotropy and dielectric aniso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com