Spherical particles of cefazolin sodium monohydrate and preparation method thereof
A technology for water cefazolin sodium and cefazolin sodium is applied in the field of cefazolin sodium spherical particles and crystallization preparation, which can solve the problems of low bulk density, rough surface, uneven particle size distribution, etc. The effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
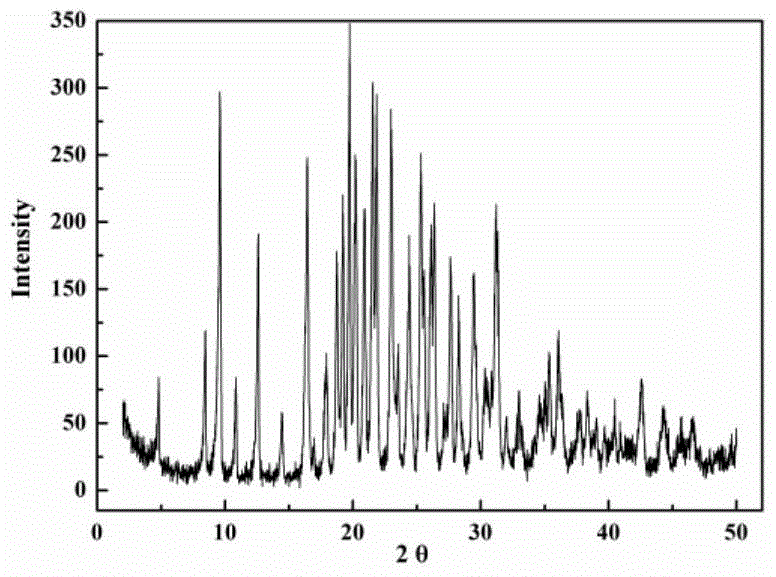
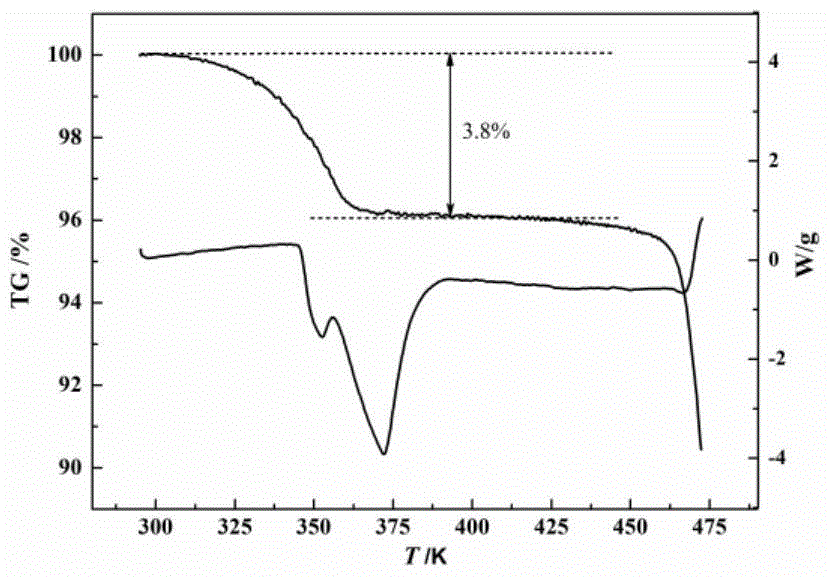
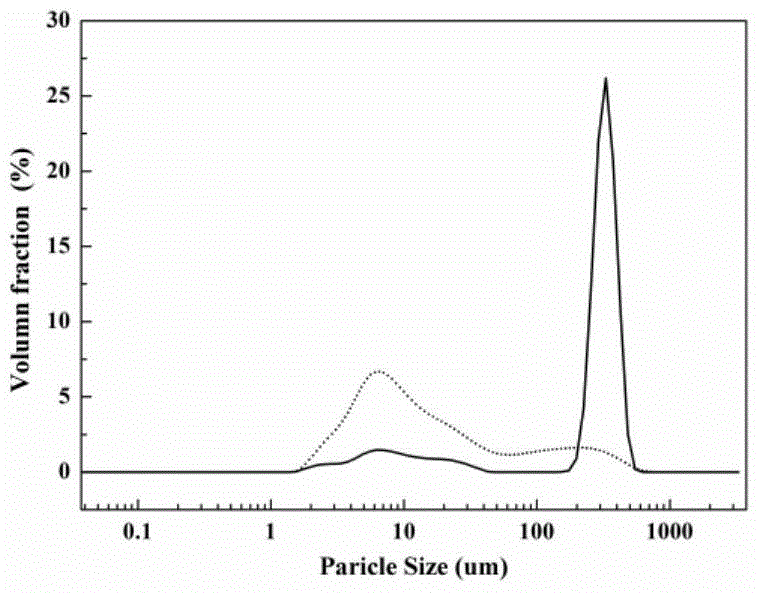
[0030] Under the condition of 40 DEG C, cefazolin sodium solid 1g is joined in the solvent system of 100g water-ethanol, wherein the mass ratio of ethanol and water is 1:1, is cooled to 5 DEG C with the cooling rate of 0.1 DEG C / min, set The stirring speed was set at 100 rpm, and 0.1% chloroform was added as a bridging agent. At this time, crystals began to precipitate. Under constant temperature and stirring conditions, the crystals grew for 5 hours. Finally, the crystal paddle was transferred to the filtration system, and the filter cake was dried at 20° C. under a vacuum of 0.1 Mpa for 10 hours to obtain spherical crystals of cefazolin sodium monohydrate, and the process yield was 95%. Product X-ray powder diffraction such as figure 1 As shown, the product TG curve is as follows figure 2 As shown, the product particle size distribution is as image 3 As shown, the appearance of the product is as follows Figure 4 and Figure 5 shown. The obtained spherical particles h...
Embodiment 2
[0032] Under the condition of 40 DEG C, cefazolin sodium solid 4g is joined in the solvent system of 100g DMAC-methanol, wherein the mass ratio of methanol and DMAC is 10:1, is cooled to 25 DEG C with the cooling rate of 5 DEG C / min, set The stirring speed was set at 250 rpm, and 0.1% toluene was added as a bridging agent. At this time, crystals began to precipitate. Under constant temperature and stirring conditions, the crystals grew for 10 hours. Finally, the crystal paddle was transferred to the filtration system, and the filter cake was dried at 35° C. for 2 hours under a vacuum of 0.1 Mpa to obtain spherical crystals of cefazolin sodium monohydrate, and the process yield was 92%. The obtained spherical particles have uniform particle size distribution, coefficient of variation C.V. = 0.28, average particle diameter equal to 302 μm, and bulk density of 0.411 g / ml. They have high sphericity, good fluidity, fast product separation speed, and pharmaceutical products have bett...
Embodiment 3
[0034]Under the condition of 40°C, cefazolin sodium solid 8g was added to the solvent system of 100g water-DMSO-n-propanol, wherein the mass ratio of n-propanol: water: DMSO was 5:0.5:0.5, and 2°C / The cooling rate of min is to cool down to 15°C, set the stirring speed to 350rpm, and add 0.3% isobutyl acetate as a bridging agent. At this time, crystals begin to precipitate. Under constant temperature and stirring conditions, the crystals grow for 8 hours. Finally, the crystal paddle was transferred to the filtration system, and the filter cake was dried at 35° C. and a vacuum of 0.1 Mpa for 6 hours to obtain spherical crystals of cefazolin sodium monohydrate, and the process yield was 94%. The obtained spherical particles have uniform particle size distribution, coefficient of variation C.V. = 0.29, average particle diameter equal to 314 μm, and bulk density of 0.42 g / ml. They have high sphericity, good fluidity, fast product separation speed, and the drug product has better fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com