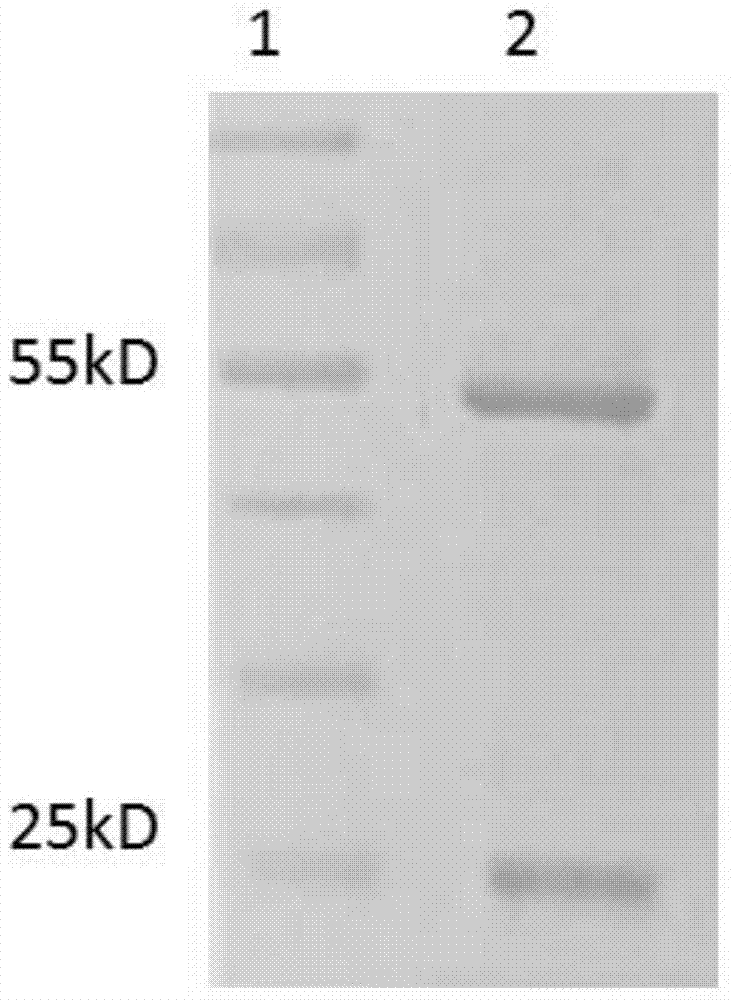
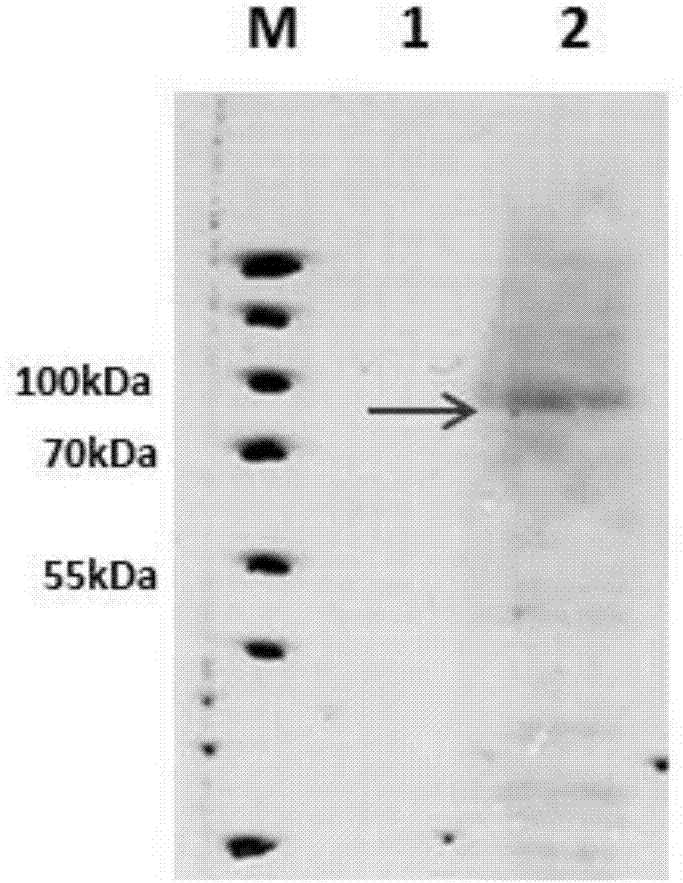
TgVP1 extracellular region antigen polypeptide, anti-TgVP1 polyclonal antibody and application of polyclonal antibody
A polyclonal antibody, antigen polypeptide technology, applied in the direction of peptide, peptide source, specific peptide, etc., can solve the problem of unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of Toxoplasma gondii TgVP1 antigen polypeptide
[0020] 1. Design and synthesis of Toxoplasma gondii TgVP1 polypeptide: The Toxoplasma gondii TgVP1 protein sequence was obtained according to the Toxoplasma gondii TgVP1 sequence on GenBank (accession number: EPT25031.1), which contains 816 amino acids.
[0021] 2. The characteristics of TgVP1 protein were analyzed by DNAstar software. The analysis results are shown in Table 1. The protein has a molecular weight of 85296 Daltons and an isoelectric point of 4.83, which is an acidic protein.
[0022] Table 1
[0023] Analysis project (Analysis)
Whole Protein
Molecular Weight
85.296kDa
Length
816aa
11.724 pMoles
Molar Extinction coefficient
95770
1A(280)=
1.10mg / mL
4.83
Charge (Charge at pH 7)
-16.1
[0024] 3. Use TMHMM software to analyze the transmembrane domain of TgVP1 pro...
Embodiment 2
[0026] Example 2 Preparation of TgVP1 Antigen Polypeptide and Coupling with Carrier Protein
[0027] 1. Peptide synthesis
[0028] In order to facilitate coupling with the carrier protein, a cysteine is added to the N-terminal of the antigenic polypeptide in the extracellular region of TgVP1, namely: CSSSPESEGDRFQVTA. The peptide was synthesized by Shanghai Jier Biochemical Company.
[0029] 2. Coupling of peptides and carrier proteins
[0030] a. Dilute one tube of maleimide-activated mcKLH with 200 μL ultrapure water to a 10 mg / mL solution. (Note: The above solution is blue-white and translucent, do not shake or heat, otherwise it will cause mcKLH to precipitate.)
[0031] b. Dissolving the thiol-containing hapten (ie TgVP1 antigenic polypeptide) with a coupling buffer equivalent to 1.0-2.5 times the volume of the solution in step a. For example: Dissolve 2 mg of hapten in 200-500 μL of coupling buffer and add to mcKLH. If the polypeptide is readily soluble, it can be...
Embodiment 3
[0043]Example 3 Preparation and Purification of Anti-TgVP1 Polypeptide Rabbit Polyclonal Antibody
[0044] 1. Animal immunization
[0045] The coupling product was used to immunize two New Zealand male rabbits, weighing 2-2.5kg. Freund's complete adjuvant or Freund's incomplete adjuvant was purchased from Sigma.
[0046] The immunization procedure is shown in Table 2. Dilute 100 μg of antigen with PBS to 1.25 mL, mix with an equal volume of adjuvant, vortex for 100 minutes, and check the emulsification result. Take a drop of antigen and drop it on water, and it will not dissolve and spread for one minute. Blood was taken from the ear vein before immunization, and serum was collected after standing at 4°C, and stored at -80°C as negative control serum.
[0047] Table 2 Specific immunization procedures
[0048]
[0049]
[0050] Antibody Titer ELISA Detection
[0051] (1) Experimental reagents
[0052] a. Phosphate buffered saline (PBS) (10×concentration): sodium chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com