Selective modification method of cane sugar primary hydroxyl
A modification method and primary hydroxyl technology, applied in the field of chemistry, can solve the problems of easy hydrolysis and cleavage, large steric hindrance, limited chemical modification, etc., and achieve the effects of not easy sucrose glycosidic bond cleavage, mild reaction conditions, and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
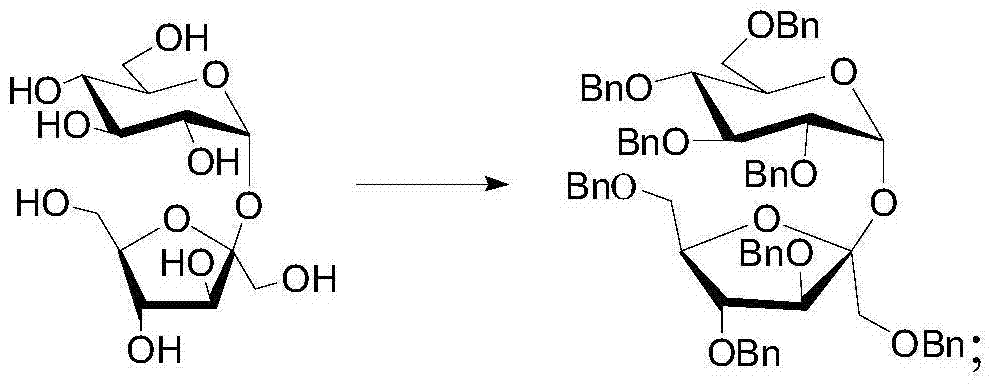
[0056] (1) After dissolving sucrose in anhydrous N,N-dimethylformamide (DMF), slowly add sodium hydride whose molar mass is 9.6 times the molar mass of sucrose, and stir at room temperature for 30 minutes, then slowly dropwise add a molar mass of Benzyl bromide with 9.6 times the molar amount of sucrose was reacted at room temperature for 24 hours. After the reaction was monitored, the reaction solution was obtained. After the reaction was quenched by slowly adding methanol dropwise to the reaction solution under an ice bath, the excess bromide was reacted for another 2 hours. Benzyl was reacted, DMF was distilled off under reduced pressure to obtain a residue, the residue was dissolved in ethyl acetate, and the insoluble matter was filtered out with a short silica gel column to obtain a filtrate. Sucrose, the yield of octabenzyl sucrose is 75%, and the reaction equation is:
[0057]
[0058] The NMR data of the product are:
[0059] 1 H NMR (400 MHz, deuterated chlorofor...
Embodiment 2
[0069] Step (1) of this embodiment is the same as step (1) of Embodiment 1;
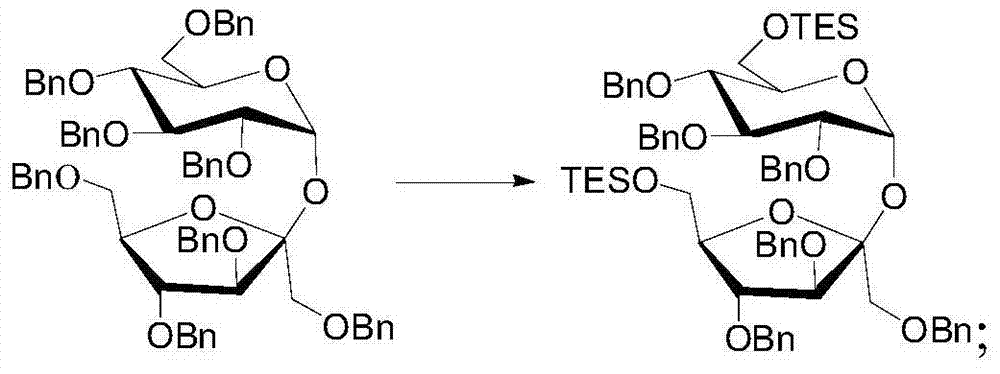
[0070](2) Dissolve octabenzyl sucrose in anhydrous benzene to obtain an octabenzyl sucrose solution with a concentration of 1mol / L. After removing the air, add the octabenzyl sucrose solution to the mixture of dicobalt octacarbonyl and triethylsilane In the solution, stir and react under reflux for 18 hours to remove the 6,6'-position dibenzyl group. The molar weight of dicobalt octacarbonyl is three times that of octabenzyl sucrose, and the molar weight of triethylsilane is octabenzyl 10 times the molar weight of sucrose. After monitoring the reaction, the reaction solution was obtained, and an appropriate amount of pyridine was added, then bubbled with air for 20 minutes, and the reaction solution was filtered through a silica gel column to obtain the filtrate. The filtrate was washed with ethyl acetate and then combined and concentrated. Chromatographic separation yielded sucrose whose 6,6′-hydrox...
Embodiment 3
[0078] Steps (1) (2) of this embodiment are the same as steps (1) (2) of Embodiment 1;
[0079] (3) Dissolve sucrose whose 6′-hydroxyl group is protected by triethylsilyl group in tetrahydrofuran, then add TBAF whose molar mass is 1.2 times the molar amount of sucrose whose 6′-position hydroxyl group is protected by triethylsilyl group, and react at room temperature After 30 minutes, the triethylsilyl group was removed. After monitoring the end of the reaction, the reaction solution was directly evaporated to dryness to obtain a residue. The residue was dissolved in ethyl acetate and filtered through a silica gel column to obtain the filtrate. The filtrate was evaporated to dryness to obtain the free Sucrose, dissolve the free sucrose at the 6′ hydroxyl group in an appropriate amount of pyridine, add an appropriate amount of acetic anhydride, and after about 3 hours of esterification, the molar amount of acetic anhydride is 5 times that of the free sucrose at the 6′ hydroxyl gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com