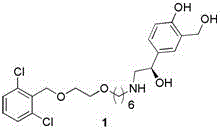
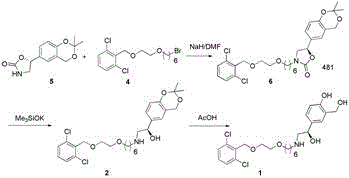
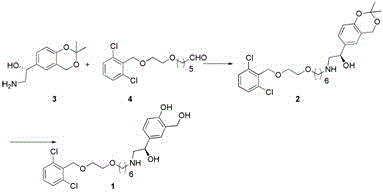
A new process for synthesizing vilanterol
A synthesis method and technology of synthesis route, which are applied in the preparation of organic compounds, preparation of aminohydroxy compounds, organic chemistry and other directions, can solve the problems of restricting industrial scale-up of vilanterol, complicated preparation, long steps and the like, and achieve short reaction steps, The effect of easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (2R)-Hydroxy-2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-N-3-[6-[2-[(2,6- Preparation of dichlorophenyl)methoxy]ethoxy]hexyl]-ethylamine
[0024] Add 300ml of methanol to a 500ml three-necked flask, and then add 31.9g of 2-({2-[(5-formyl-hexyloxy)]-ethoxy}methyl)-1,3-dichlorobenzene With 22.3g of (2R)-hydroxyl-2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-ethylamine, stirred at room temperature for about 5h, and the reaction was completed by TLC monitoring. Slowly add 4.2 g of sodium borohydride in batches, and control the internal temperature not to exceed 30°C. After the addition was complete, 5ml of water was slowly added dropwise to quench the reaction. Stir and add the reaction solution to 900ml of water, stir for 30min, add isopropyl ether to extract 3 times, wash with saturated brine 3 times, dry over anhydrous sodium sulfate, dry and concentrate under reduced pressure, the residue is purified by column chromatography to obtain (2R)- Hydroxy-2-(2,2-dimethyl-4H-1,3-ben...
Embodiment 2
[0026] 4-{(1R)-2-[(6-{2-[(2,6-dichlorophenyl)methoxy]ethoxy}hexyl)amine]-1-hydroxyethyl}-2- ( Preparation of hydroxymethyl)phenol (vilanterol)
[0027] (2R)-Hydroxy-2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-N-3-[6-[2-[(2,6 Add 46.4 g of -dichlorophenyl)methoxy]ethoxy]hexyl]-ethylamine to 200ml of acetic acid, then add 100ml of water, stir at room temperature for 7-8h, and monitor the completion of the reaction by TLC. Concentrate under reduced pressure, add 400ml of dichloromethane to the residue, adjust the pH to 7-8 with saturated sodium bicarbonate, separate the layers, wash the organic phase once with saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. Chromatographic purification afforded 39.5 g of vilanterol with a yield of about 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com