Application of chlorogenic acid in preparation of medicines for treating pathologic jaundice
A technology of pathological jaundice and chlorogenic acid, applied in the field of biomedicine, to achieve the effect of inhibiting the release and promoting the biosynthesis of glutathione
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 In vivo pharmacodynamic experiment of chlorogenic acid on pathological jaundice
[0025] 1. Experimental materials and methods
[0026] 1.1 Experimental animals
[0027] 65 clean-grade adult SD rats, half male and half male, weighing 200g to 230g
[0028] 1.2 Experimental drugs and reagents
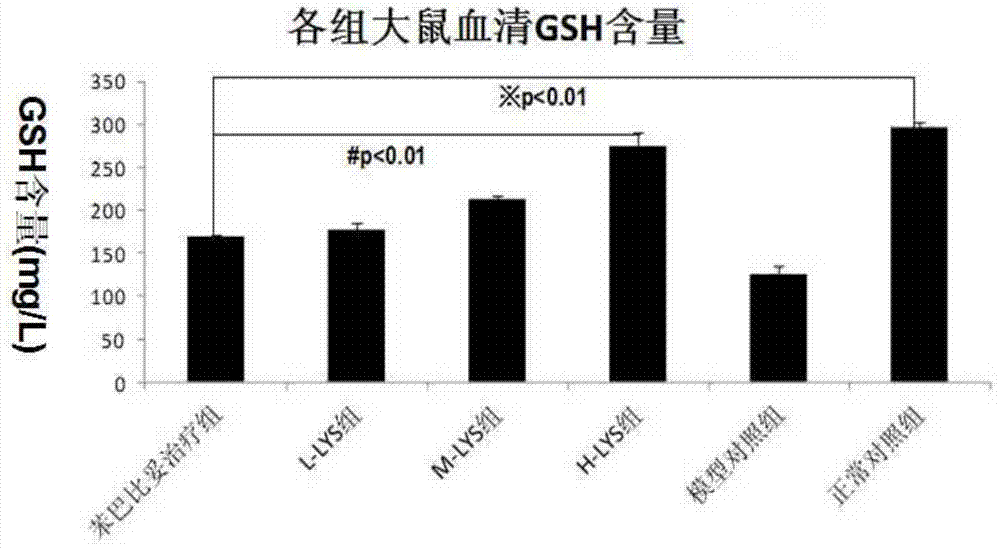
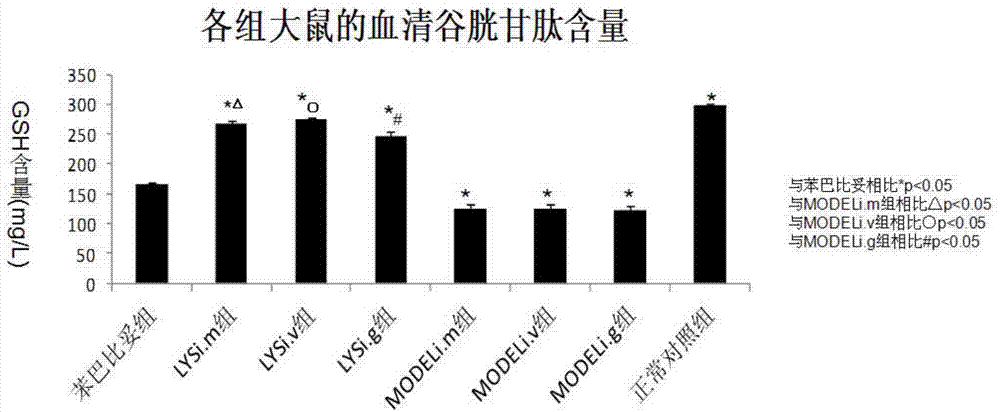
[0029] Chlorogenic acid, phenobarbital sodium injection (in the experiment, chlorogenic acid or phenobarbital sodium can be dissolved in normal saline according to the dosage to make a series of chlorogenic acid and phenobarbital sodium with different concentrations Intraperitoneal injection for spare), total bilirubin assay kit, direct bilirubin assay kit, glutathione assay kit, one ten thousandth electronic balance.
[0030] 1.3 Establishment of animal models and group administration
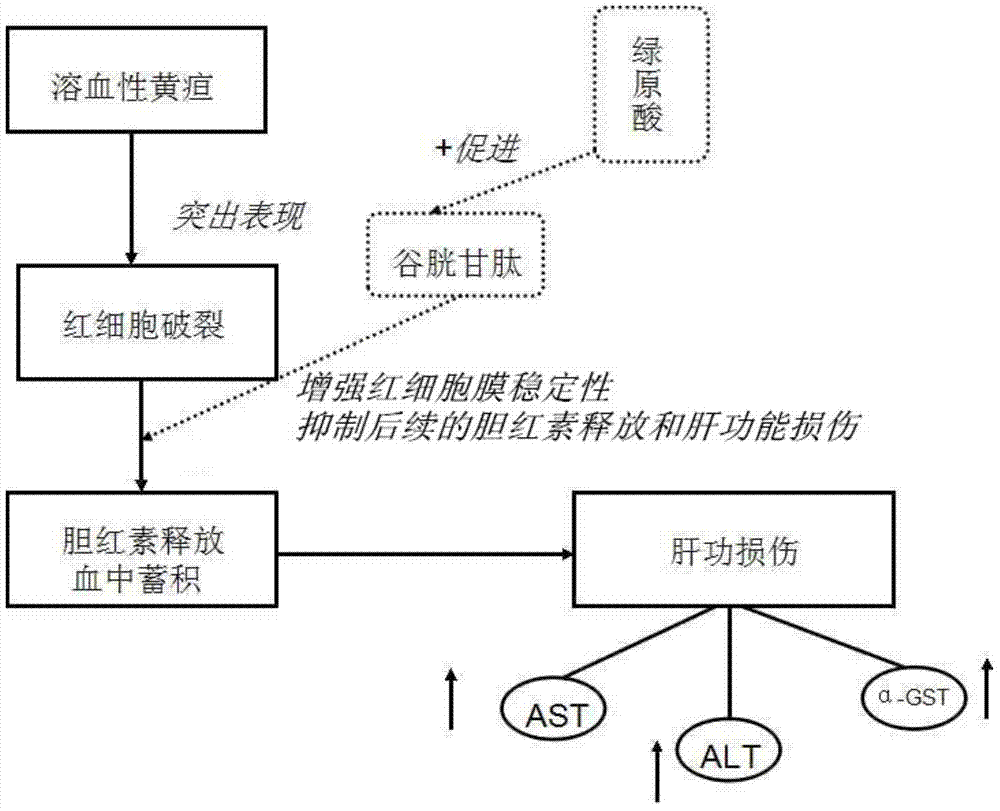
[0031] In this example, acetylphenylhydrazine (APH) was used as a modeling drug to construct a rat model of hemolytic jaundice, which is also a commonly used model for studying neonat...
Embodiment 2
[0061] Taking chlorogenic acid as an active ingredient and adding one or more pharmaceutically acceptable excipients to prepare drugs in different dosage forms such as oral preparations, injection preparations or external transdermal preparations. The content of chlorogenic acid per preparation unit in the obtained medicine is controlled to be 1-3000 mg.
[0062] The in vivo pharmacodynamic experiment of chlorogenic acid on pathological jaundice was carried out according to the experimental method described in Example 1. The difference is: respectively change the way of administration to three ways of intramuscular injection, intravenous injection and intragastric administration to carry out the experiment. The results show that the above four administration methods can effectively reduce α-GST, ALT, AST, direct bilirubin and indirect bilirubin in the serum of pathological jaundice model mice and promote the synthesis of glutathione in the experimental group mice .
[0063] ...
Embodiment 3
[0102] Embodiment 3: Prepare freeze-dried powder injection with chlorogenic acid
[0103] 1. Extraction of chlorogenic acid:
[0104] The chlorogenic acid raw material drug used in this example is obtained by extracting and purifying the burdock fruit with a purity of 99.44%.
[0105] 2. Preparation of chlorogenic acid freeze-dried powder injection
[0106] 2.1 Prescription:
[0107]
[0108] Add the above prescription into water for injection, stir until it is completely dissolved, adjust the pH, filter it with a 0.22 μm sterile microporous filter membrane, and make a total of 2000 2ml powder injections according to the routine operation of sterile freeze-dried powder injection, each Contains chlorogenic acid 50mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com