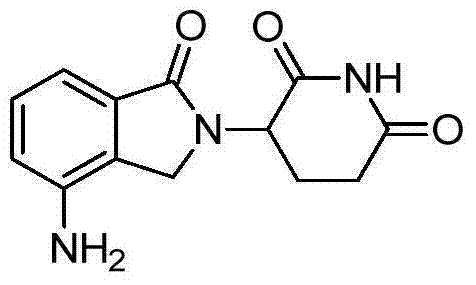
3-(4-amino-1, 3-dihydro-1-oxo-2 H-isoindole-2-yl)-2, 6-piperidinedione preparation method
A technology of hydrogen and dimethyl sulfoxide, which is applied in the field of synthesis of 3--2,6-piperidinedione, can solve the problems of high equipment requirements, high risk factor, and difficult purification, and achieve low equipment requirements and low cost. The effect of low and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
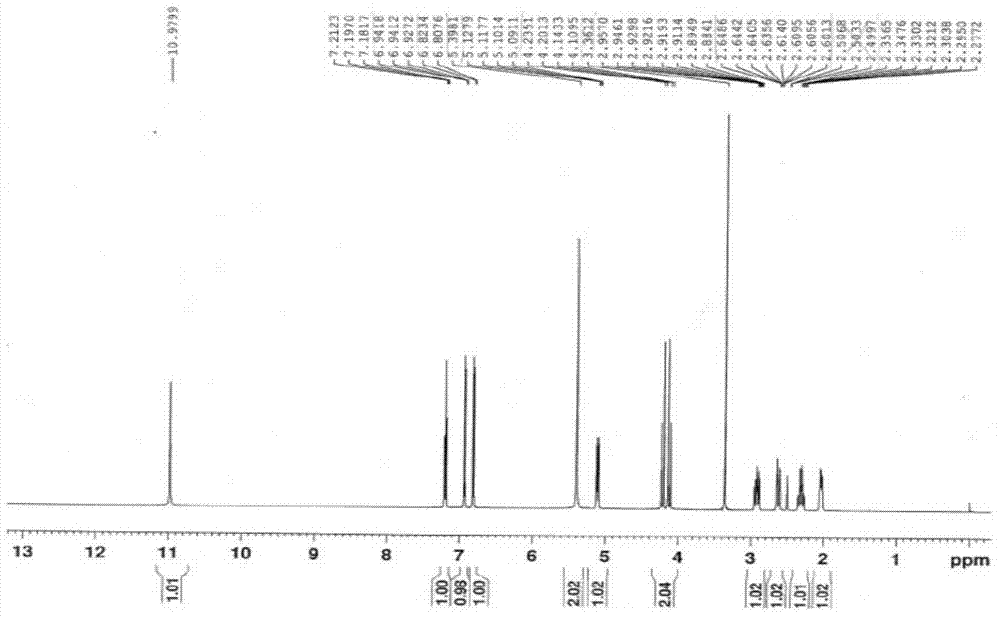
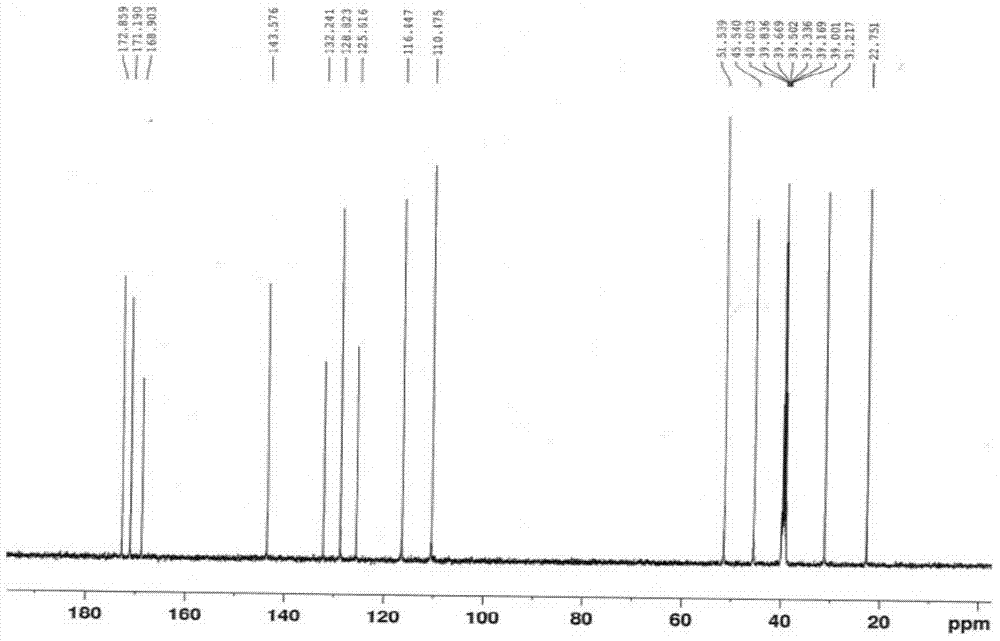
[0023] Compound II (3-(4-nitro-1,3-dihydro-1-oxo-2H-isoindol-2-yl)piperidine-2,6-dione) (28.9g, 0.1mol ), 10% palladium carbon (10.0g), and ammonium formate (15.8g, 0.25mol) were added to dimethyl sulfoxide (500ml), kept at a temperature of 30-40°C, reacted for 7-8h, filtered while hot, and the filtrate was added dropwise Put it into water (1500ml), stir and crystallize, filter with suction, wash the filter cake with water, and dry the filter cake in vacuum at room temperature for 14-16 hours to obtain compound I (light yellow solid, 24.2g), molar yield: 93.4%. Melting point: 269-271°C, HPLC: 99.94%. LC / MS (m / c): 260.10 (MH + ), the product was determined to be compound I after NMR structure analysis, and its hydrogen spectrum was as follows figure 1 As shown, the carbon spectrum as figure 2 shown.
Embodiment 2
[0025] Compound II (3-(4-nitro-1,3-dihydro-1-oxo-2H-isoindol-2-yl)piperidine-2,6-dione) (14.5g, 0.05mol ), 10% palladium carbon (5.0g), and ammonium formate (7.88g, 0.125mol) were added to methanol (700ml), kept at a temperature of 30-40°C, reacted for 7-8h, filtered while hot, concentrated the filtrate to 100ml, added water (500ml) was beaten for 1 hour, filtered, and the filter cake was vacuum-dried at room temperature for 14-16 hours to obtain compound I (yellow solid, 8.43g), molar yield: 65%. The product was identified as compound I after NMR structure analysis.
Embodiment 3
[0027] Compound II (3-(4-nitro-1,3-dihydro-1-oxo-2H-isoindol-2-yl)piperidine-2,6-dione) (14.5g, 0.05mol ), 10% palladium carbon (5.0g), and ammonium formate (7.88g, 0.125mol) were added to N-methylpyrrolidone (200ml), kept at a temperature of 30-40°C, reacted for 7-8h, filtered while hot, and the filtrate dripped Add it into water (1000ml), stir and crystallize, filter with suction, wash the filter cake with water, and dry the filter cake under vacuum at room temperature for 14-16 hours to obtain compound I (light yellow solid, 11.8g), molar yield: 90.8%. The product was identified as compound I after NMR structure analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com