Preparations containing ceftezole sodium
A technology of ceftezole sodium and aerosol, which is applied in the field of aerosol containing ceftezole sodium, can solve the problems of inconvenient administration of ceftezole sodium, and achieve good hygroscopicity, improved stability, and good sprayability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Ceftezole Sodium: 5 parts
[0025] Ethanol: 450 parts
[0026] Polysorbate 40:8 parts
[0027] Lauryl alcohol: 5 parts
[0028] f 12 : 550 copies.
[0029] Preparation:
[0030] (1) dissolve ceftezole sodium with ethanol to obtain a clear and uniform solution;
[0031] (2) take polysorbate 40 and lauryl alcohol of recipe quantity respectively, add in the above-mentioned solution, make solution system;
[0032] (3) Put the solution obtained in step (2) into the container, install the valve and tighten it, and press the propellant F 12 , Shake well to get an aerosol.
Embodiment 2
[0084] Embodiment 2 Ceftezole Sodium Aerosol Accelerated Test
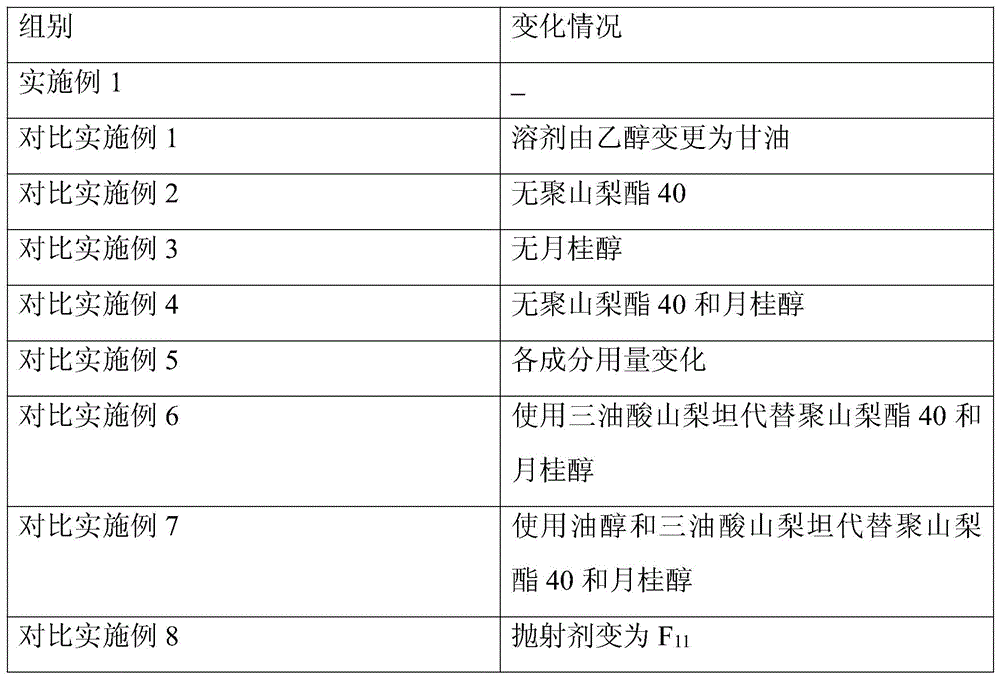
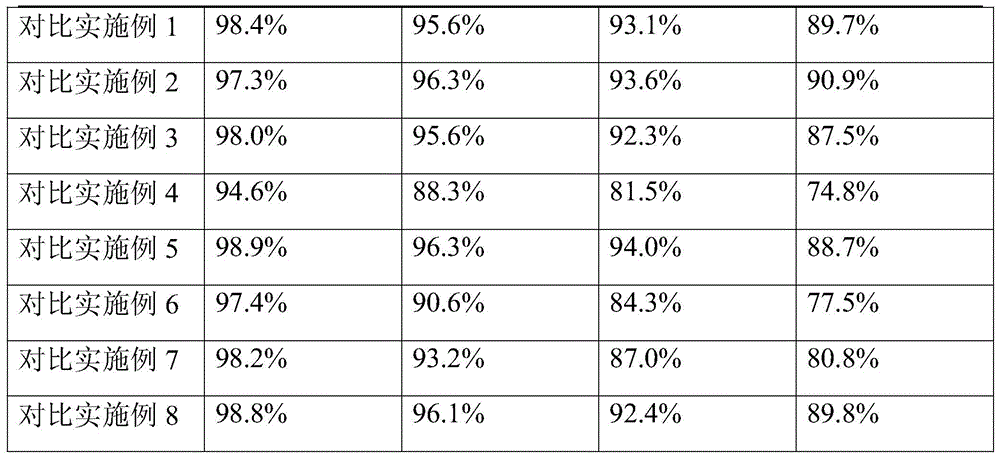
[0085] Accelerated test: Get the samples of Ceftezole Sodium Aerosol of the present invention Example 1 and Comparative Examples 1-8, place in DHS-100 constant temperature and humidity chamber (Beijing Yashilin Experimental Equipment Co., Ltd.), adjust the temperature to 40°C , relative humidity is 75%, respectively sampling once in 1,2,3,6 months, detects ceftezole sodium content (percentage by weight), the results are shown in Table 1.
[0086]Table 1 Accelerated test results of ceftezole sodium aerosol
[0087] Table 1 Accelerated test results
[0088]
[0089]
[0090] As can be seen from Table 1, the stability of the aerosol of Example 1 is the best, no matter changing the aerosol adjuvant or the dosage, all can significantly reduce the stability of the ceftezole sodium aerosol.
Embodiment 3
[0091] Example 3 Ceftezole Sodium Aerosol Room Temperature Sample Observation Result
[0092] According to Chinese Pharmacopoeia 2000 edition two, carry out visual observation and analysis of aerosol, the results are shown in Table 2.
[0093] Table 2
[0094]
[0095] According to Table 2, it can be seen that the aerosol prepared by ethanol solution is the most stable, and the aerosol solution prepared with polysorbate 40 and lauryl alcohol as co-solvents has the best sensory in all aspects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com