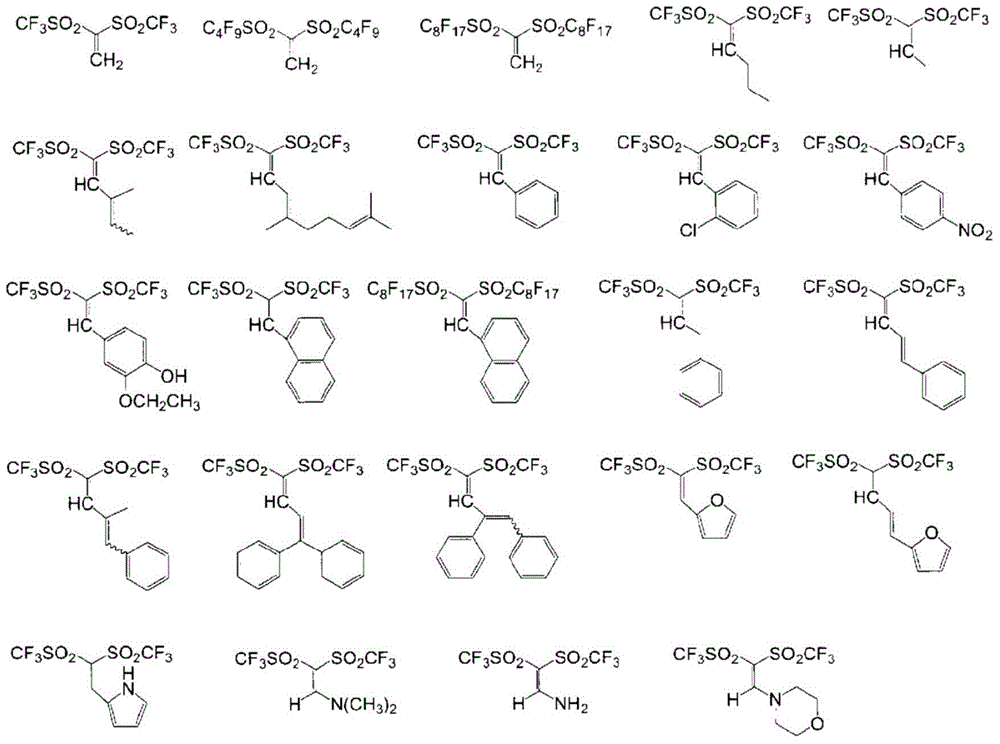
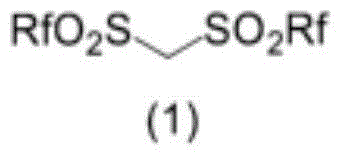
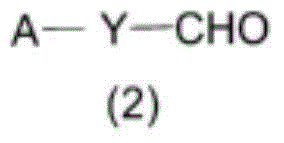Method for producing compound containing bis(perfluoroalkylsulfonyl)methyl group and salt thereof, and solid electrolyte membrane produced using same
一种制造方法、化合物的技术,应用在固体电解质、有机化合物的制备、固体电解质燃料电池等方向,能够解决过量使用、化合物不稳定、原料化合物不容易合成等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0251] [Production of bis(trifluoromethanesulfonyl)heptane using phenylsilane as a reducing agent]
[0252] The reaction formula of this production method is shown below.
[0253]
[0254] After dissolving 1.1 g of hexanal in 10 ml of dichloromethane, 2.8 g of bis(trifluoromethanesulfonyl)methane was added as the compound (1) in Invention 1, and stirred at room temperature for 15 minutes. Next, 1.1 g of phenylsilane was added as a reducing agent as compound (4), and stirred at room temperature for 14 hours. Magnesium sulfate 1.0 g was added to the reaction liquid after stirring, and it filtered after dehydrating and drying. After the filtrate was concentrated under reduced pressure, it was purified by distillation at a temperature of 90° C. and a pressure of 1.33 Pa to obtain 2.7 g of bis(trifluoromethanesulfonyl)heptane as a compound (5) in a yield of 75%. The measurement results of nuclear magnetic resonance spectroscopy (NMR) are shown below.
[0255] Colorless liquid...
Embodiment 2
[0257] [Production of bis(trifluoromethanesulfonyl)heptane using phenyldimethylsilane as a reducing agent]
[0258] In place of phenylsilane, the compound (4) of Example 1, phenyldimethylsilane having a strong reducing power was used. The reaction formula of this production method is shown below.
[0259]
[0260] 2.8 g of bis(trifluoromethanesulfonyl)methane as the compound (1) was added to a solution of 1.1 g of hexanal in 10 ml of dichloromethane at room temperature, followed by stirring for 15 minutes. To the reaction solution, 1.5 g of phenyldimethylsilane was added as a compound (4), followed by stirring at room temperature for 14 hours. Using 151 mg of trifluoromethylbenzene as an internal standard substance, the reaction solution was 19 According to F-NMR measurement, bis(trifluoromethanesulfonyl)heptane was obtained as compound (5) in an NMR yield of 32%.
[0261] When phenyldimethylsilane having a strong reducing power was used instead of the phenylsilane of Ex...
Embodiment 3
[0268] [production of bis(trifluoromethanesulfonyl)heptane using acetal compound]
[0269] The reaction formula of this production method is shown below.
[0270]
[0271] After dissolving 174 mg of 1,1-diethoxyhexane as compound (3) in 1.0 ml of toluene, 116 mg of triethylsilane as compound (4) was added as a reducing agent, and stirred at room temperature for 15 minutes. To the reaction solution was added 280 mg of bis(trifluoromethanesulfonyl)methane as compound (1), followed by stirring at room temperature for 12 hours. After adding 95 mg of trifluoromethylbenzene as an internal standard substance to the stirred reaction liquid, carry out 19 F-NMR determination. The yield was calculated from the ratio of the integrated value of the internal standard substance, and compound (5) bis(trifluoromethanesulfonyl)heptane was obtained at a yield of 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent porosity | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com