Method for producing extended-release potassium citrate wax matrix tablet
A technology of potassium citrate tablets and potassium citrate, which can be used in pill delivery, pharmaceutical formulations, non-active ingredients of oil/fat/wax, etc., and can solve problems for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
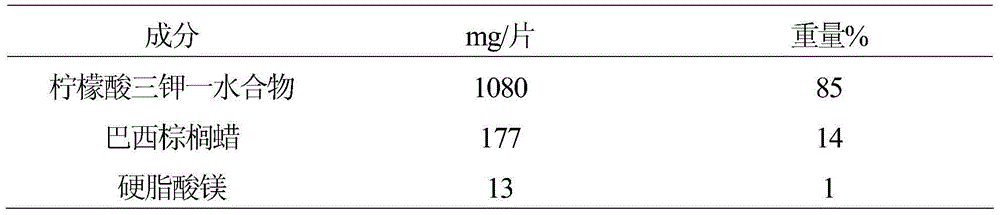
[0018] The 10-mg equivalent tablets were prepared by drying the combined potassium citrate and carnauba wax. The recipe is shown in Table 3.
[0019] table 3
[0020]
[0021] Potassium citrate was sieved through a 18 screen and then mixed with carnauba wax in a Sigma mixer for 5 minutes. Magnesium stearate sieved through a 30 sieve was added to the potassium citrate-carnauba wax mixture and mixed for 1 minute. The granules were compressed into 18.9x8.6mm oval tablets in a Stokes-Pennwalt rotary tablet press model 900. The tablet hardness was 7kp and the tablet had an abrasion of 1.8%, which was unacceptable. The dissolution profile is as follows:
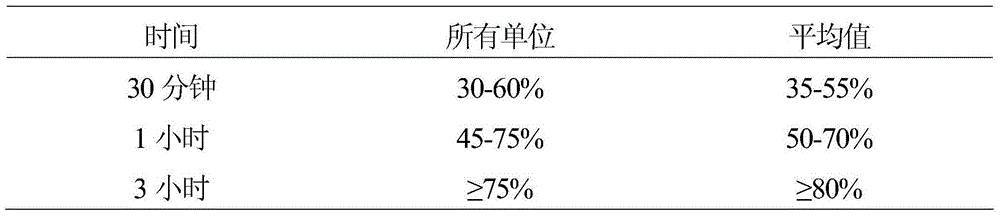
[0022] Table 4 (Solubility, 12 units)
[0023]
[0024] The above products do not meet the dissolution requirements of USP 35. This example illustrates that dry blending of 14% by weight carnauba wax used to produce granules for direct compression does not produce tablets meeting the USP requirements for potassium citra...
Embodiment 3
[0026] The 10-meq tablets were prepared by dry blending potassium citrate and carnauba wax. The formula is shown in Table 5.
[0027] table 5
[0028]
[0029] Sift through a 18 sieve of potassium citrate, then blend with carnauba wax in a Sigma mixer for 5 minutes. Magnesium stearate sieved through a 30 sieve was added to the potassium citrate-carnauba wax mixture and mixed for 1 minute. The granules were compressed into 18.9x8.6mm oval tablets in a Stokes-Pennwalt rotary tablet press model 900. The tablet hardness was 7kp and the abrasion of the tablet was 2%, which was not acceptable. The dissolution profile is as follows:
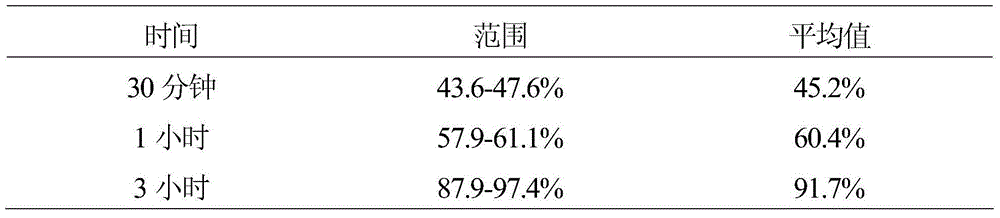
[0030] Table 6 (Solubility, 12 units)
[0031]
[0032] This example illustrates that dry blending of 20% by weight carnauba wax for the production of granules for direct compression, while meeting compendial solubility requirements, did not produce tablets with acceptable abrasion.
Embodiment 4
[0034] 10-mequivalent tablets were prepared using the same formulation as in Example 2 by sufficiently melting carnauba wax. The process is as follows:
[0035] 1. Crush the potassium citrate with a knife forward in the Fitzmill D6, using a perforated screen 8.
[0036] 2. Mix the crushed potassium citrate from #1 with carnauba wax in a sigma mixer for 20 minutes.
[0037] 3. In the Fitzmill D6, knives forward to crush the granules from #2, using a perforated screen 12.
[0038] 4. In a jacketed Sigma mixer, heat the pellets from #3 with constant mixing. Continue heating until the carnauba wax is completely melted (above 80°C), and thereafter for an additional 10 minutes.
[0039] 5. Pour the liquid mass from #4 into a 2"x 2"x 2" mold and allow to cool to room temperature.
[0040] 6. In Fitzmill D6, knives forward to break up lumps from #5, using perforated screen 16.
[0041] 7. Sift the magnesium stearate through a sieve 30 and blend with the crushed granules from #6 i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com