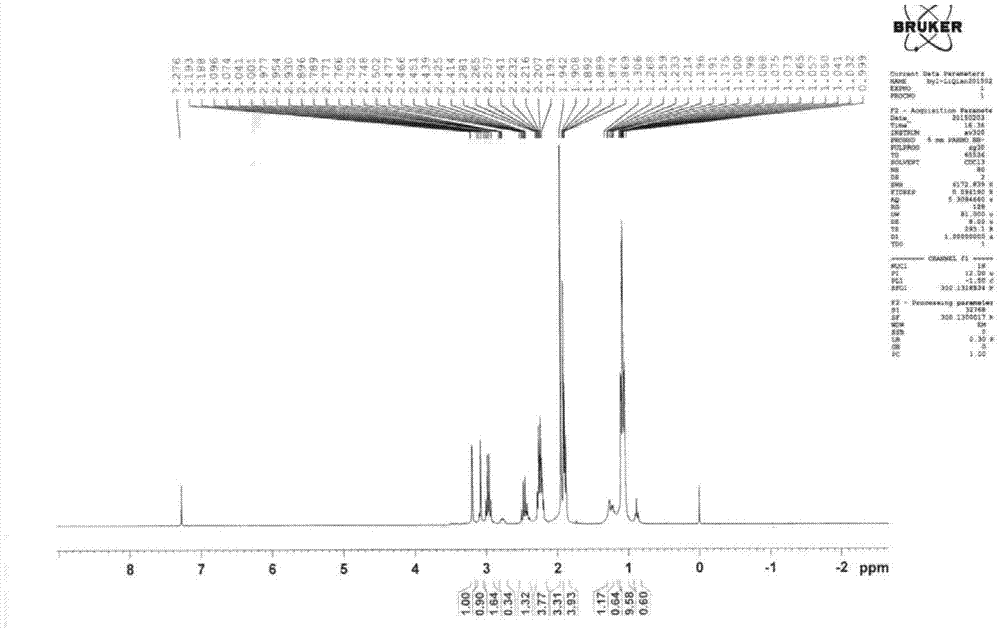
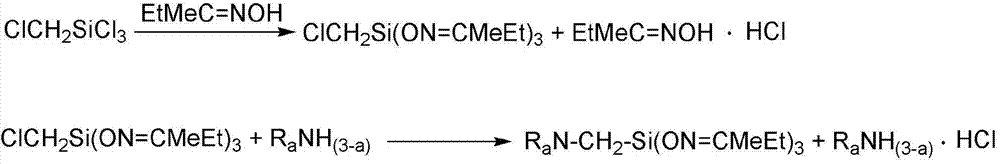A kind of preparation method of α-aminotributanone oxime base silane
A technology of aminotributanone oxime and chloromethyl tributanone oxime, which is applied in the field of organic synthesis, can solve the problems of unsatisfactory reaction conversion rate, large amount of amine, difficult complete reaction of alkoxy groups, etc., and achieve multi-substitution The effects of less by-product formation, good adhesion and thermal stability, and easy control of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of α-(β-aminoethyl) aminomethyl tributylketoxime base silane, comprises steps as follows:
[0037] (1) Mix 132.86g butanone oxime and 216.0mL petroleum ether evenly, add 46.0g chloromethyltrichlorosilane evenly within 40 minutes, after the addition is completed, react at 25°C for 4 hours, pour into Stand in a dry separatory funnel for stratification, neutralize the lower liquid with triethylamine to recover butanone oxime, neutralize the supernatant with triethylamine, filter off the insoluble triethylamine hydrochloride, and distill under reduced pressure at 6mmHg , distill off the organic solvent petroleum ether and the remaining reactants butanone oxime and triethylamine to obtain chloromethyl tributylketoxime base silane;
[0038] (2) Mix 60.00g of ethylenediamine and 135mL of petroleum ether evenly, and then uniformly dropwise add the chloromethyltributylketoximosilane obtained in step (1) within 60min. After the addition is completed, ...
Embodiment 2
[0041] A kind of preparation method of α-(N,N-diethyl) aminomethyl tributylketoxime base silane, comprises steps as follows:
[0042](1) Mix 105.42g butanone oxime and 115.0mL n-hexane evenly, and add 36.78g chloromethyltrichlorosilane evenly within 40 minutes. After the addition is completed, react at 20°C for 3 hours, and pour into Stand in a dry separatory funnel for stratification, neutralize the lower liquid with triethylamine to recover butanone oxime, neutralize the supernatant with triethylamine, filter off the insoluble triethylamine hydrochloride, and distill under reduced pressure at 5mmHg , distill off the organic solvent n-hexane and the remaining reactants butanone oxime and triethylamine to obtain chloromethyl tributylketoxime base silane;
[0043] (2) Mix 36.57g of diethylamine and 52mL of n-hexane evenly, and then uniformly dropwise add the chloromethyltributylketoximosilane obtained in step (1) within 40min. After the addition is completed, react at 40°C Aft...
Embodiment 3
[0047] A preparation method of α-(N,N-di-n-butyl)aminomethyltributanoximinosilane, comprising the following steps:
[0048] (1) Mix 53.58g of butanone oxime and 145.0mL of n-hexane evenly, add 18.41g of chloromethyltrichlorosilane uniformly within 20 minutes, after the addition is completed, react at 30°C for 4 hours, pour into Stand in a dry separatory funnel for stratification, neutralize the lower liquid with pyridine to recover butanone oxime, neutralize the supernatant with pyridine, filter off the insoluble matter tripyridine hydrochloride, distill under reduced pressure at 7 mmHg, and evaporate the organic solvent n-hexane and remaining reactant butanone oxime and pyridine, get chloromethyl tributylketoxime base silane;
[0049] (2) Mix 38.81g of di-n-butylamine and 105mL of n-hexane evenly, and then uniformly dropwise add the chloromethyltributanoximinosilane obtained in step (1) within 25min. After the addition is completed, at 50°C, React for 5 hours. After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com