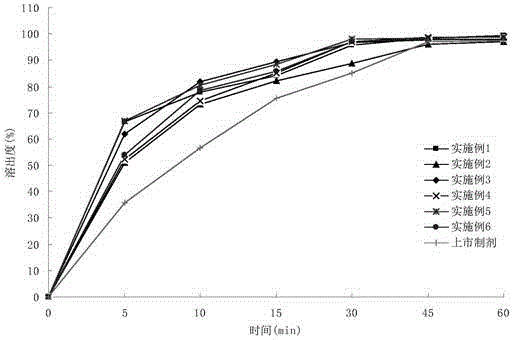
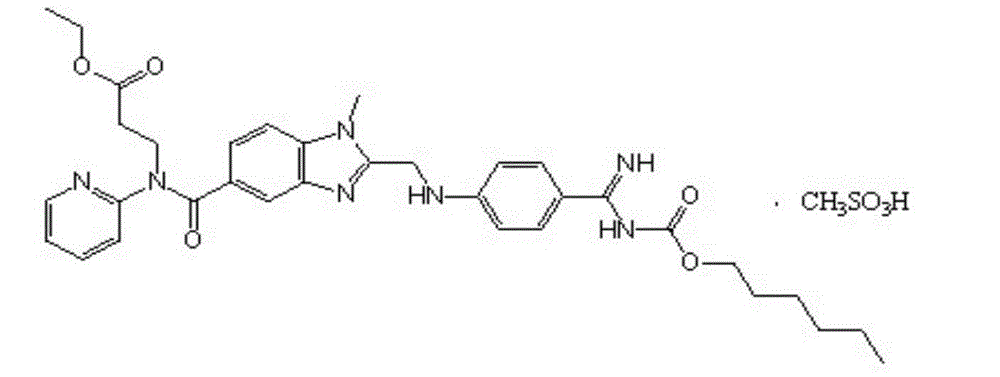
Pradaxa composition
A technology for dabigatran etexilate mesylate and a composition is applied in the field of pharmaceutical compositions containing dabigatran etexilate mesylate and the field of preparation thereof, and can solve the problems of numerous production processes, difficulty in industrialized production, and methanesulfonic acid. Problems such as complex production process of dabigatran etexilate capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Preparation:
[0026] (1) Micronize dabigatran etexilate mesylate to make D 90 Below 20μm.
[0027] (2) Dissolve the prescribed amount of sodium lauryl sulfate and povidone K30 in the prescribed amount of purified water, and use it as an adhesive for later use.
[0028] (3) Mix the prescribed amount of dabigatran etexilate mesylate, lactose, and microcrystalline cellulose evenly, add a binder to make a soft material, granulate, dry, and granulate.
[0029] (4) Compressed tablets, each containing 50 mg of dabigatran etexilate mesylate (as dabigatran etexilate).
Embodiment 2
[0031] The granules prepared in Example 1 are packed into capsules, each containing dabigatran etexilate mesylate 50mg (as dabigatran etexilate).
Embodiment 3
[0033]
[0034] Preparation:
[0035] (1) Micronize dabigatran etexilate mesylate to make D 90 Below 20μm.
[0036] (2) Dissolve the prescribed amount of poloxamer and povidone K30 in purified water and use it as an adhesive for later use.
[0037] (3) Mix the prescription amount of dabigatran etexilate mesylate, lactose, and pregelatinized starch evenly, add binder to make soft material, granulate, dry, and granulate.
[0038] (4) Compressed tablets, each containing 50 mg of dabigatran etexilate mesylate (as dabigatran etexilate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com