A kind of sustained-release azithromycin lactobionate injection and preparation method thereof
A technology of azithromycin and lactobionic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of reducing the number of medications, uniform particle size distribution, and prolonging the efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
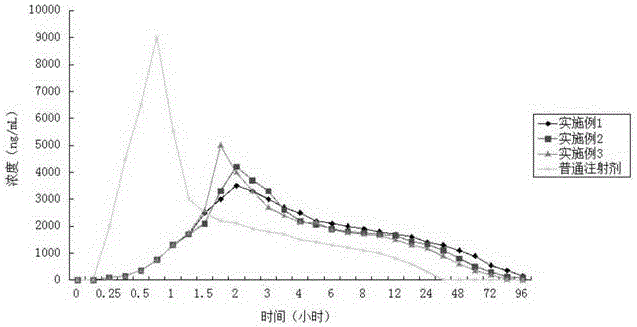
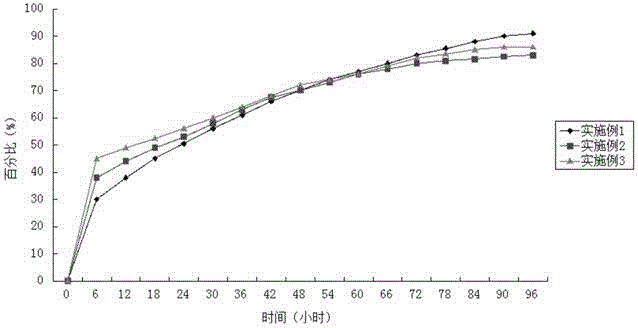
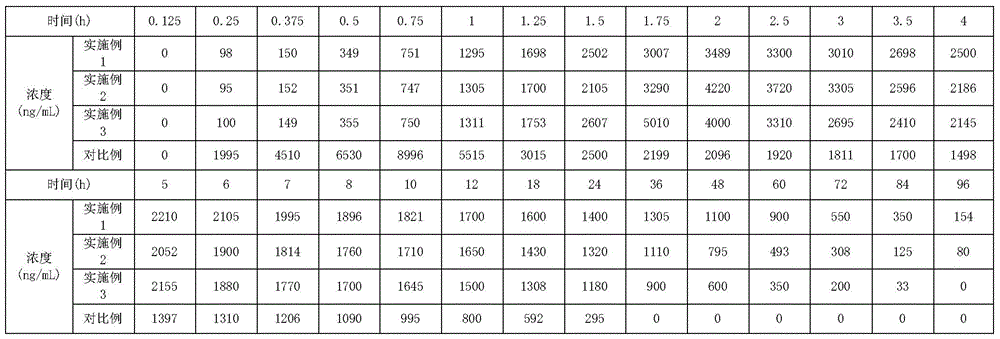
Image
Examples
Embodiment 1
[0033] Weigh 50g of azithromycin lactobionate, 115g of polybenzyl glutamate, and 35g of polyhydroxybutyric acid, dissolve them in chloroform, control the solid concentration of the mixed solution at 35% by weight, stir and add to the polyvinyl alcohol stirred at a speed of 5500RPM In the aqueous solution (viscosity at room temperature is 40mPa·s), continue to stir to form O / W emulsion droplets, and then stir continuously at room temperature at a speed of 800RPM to evaporate the solvent. After the system is solidified, wash with water and dry in vacuum at room temperature to obtain powdery Balls, sterilized;
[0034] Dissolve 15g of mannitol, 8g of carboxymethylcellulose sodium and 1g of Tween-80 in 2000mL of sterile water; centrifuge to remove insoluble matter to prepare a suspension matrix for injection; Suspend the spheres in the suspension matrix, and stir evenly; fill the suspension containing the microspheres into a sterilized vial under stirring; freeze-dry the filled su...
Embodiment 2
[0036] Weigh 50g of azithromycin lactobionate, 130g of polybenzyl glutamate, 20g of polyhydroxybutyric acid, dissolve in chloroform, control the solid concentration of the mixed solution at 35% by weight, stir and add to the polyvinyl alcohol stirred at a speed of 5500RPM In the aqueous solution (viscosity at room temperature is 40mPa·s), continue to stir to form O / W emulsion droplets, and then stir continuously at room temperature at a speed of 800RPM to evaporate the solvent. After the system is solidified, wash with water and dry in vacuum at room temperature to obtain powdery Balls, sterilized;
[0037] Dissolve 15g of mannitol, 8g of carboxymethylcellulose sodium and 1g of Tween-80 in 2000mL of sterile water; remove the insoluble matter by filtration to obtain a suspension matrix for injection; Suspend the microspheres in the suspension matrix and stir evenly; fill the suspension containing the microspheres into a sterilized vial under stirring; freeze-dry the filled susp...
Embodiment 3
[0039] Weigh 50g azithromycin lactobionate, 100g polybenzyl glutamate, 50g polyhydroxybutyric acid, dissolve in chloroform, control the solid concentration of the mixed solution at 35% by weight, stir and add to the polyvinyl alcohol stirred at a speed of 5500RPM In the aqueous solution (viscosity at room temperature is 40mPa·s), continue to stir to form O / W emulsion droplets, and then stir continuously at room temperature at a speed of 800RPM to evaporate the solvent. After the system is solidified, wash with water and dry in vacuum at room temperature to obtain powdery Balls, sterilized;
[0040] Dissolve 15g of mannitol, 8g of carboxymethylcellulose sodium and 1g of Tween-80 in 2000mL of sterile water; centrifuge to remove insoluble matter to prepare a suspension matrix for injection; Suspend the spheres in the suspension matrix, and stir evenly; fill the suspension containing the microspheres into a sterilized vial under stirring; freeze-dry the filled suspension, And det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com