Organic iridium metal complex as well as preparation method and application thereof
An iridium metal complex, organic technology, applied in indium organic compounds, platinum group organic compounds, organic chemistry and other directions, can solve the problem of low color purity of red light, and achieve the effect of improving light purity, improving luminous efficiency and reducing accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
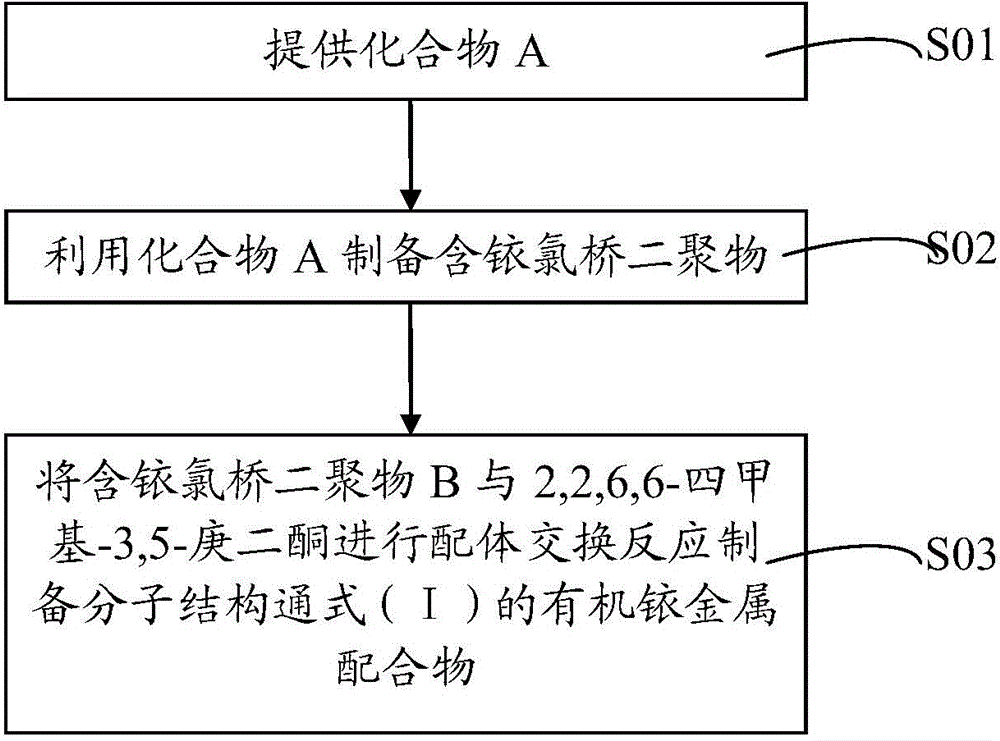
[0037] Correspondingly, the embodiment of the present invention also provides a method that can be used to prepare the organic iridium metal complex of the general molecular structure formula (I), the preparation method flow is as follows figure 1 shown. The preparation method of the organic iridium metal complex comprises the following steps:
[0038] S01. Compound A providing the following general structural formula,
[0039]
[0040] S02. In an oxygen-free environment, the compound A and iridium trichloride hydrate are heated and reacted in a reaction solvent to generate an iridium-containing chlorine bridge dimer B of the following general structure;
[0041]
[0042] S03. In an oxygen-free environment, the above-mentioned iridium-containing chlorine bridge dimer B and the auxiliary ligand source 2,2,6,6-tetramethyl-3,5-heptanedione in a reaction solvent containing a basic catalyst Heating reaction in the middle, purify, obtain the organic iridium metal complex of ...
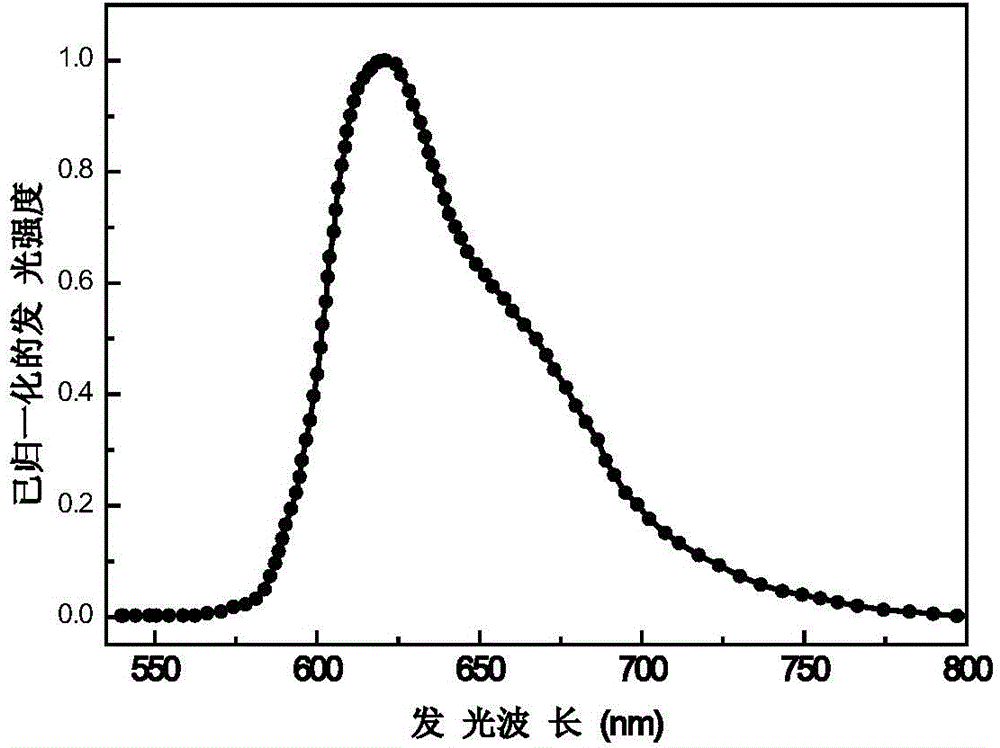
Embodiment 1
[0094] Bis[1-(9',9''-spirobifluoren-2'-yl)isoquinoline-N,C3'](2,2,6,6-tetramethyl-3,5-heptanedione ) iridium metal complex and preparation method thereof
[0095] (1) Synthesis of 2-pinacol borate-9,9'-spirobifluorene
[0096] In an argon atmosphere, 5.63 mL of a 1.6 M n-butyllithium (9 mmol) n-hexane solution was added to a solution containing 2.37 g (6 mmol) of 2-bromo-9,9'-spirobifluorene and 40 mL of dry tetrahydrofuran ( THF) in a 100 mL two-necked flask, the mixture was stirred at -78°C for 30 min. Add 1.84mL (1.67g, 9mmol) of isopropanol pinacol borate, and continue to stir the reaction for 2h after the reaction system naturally rises to room temperature. Add 20 mL of distilled water to terminate the reaction, extract 3 times with 50 mL of diethyl ether, and combine the organic phases. The organic phase was washed with saturated brine and dried over anhydrous magnesium sulfate. Filtrate, and rotary evaporate the solvent in the filtrate to obtain the crude product. ...
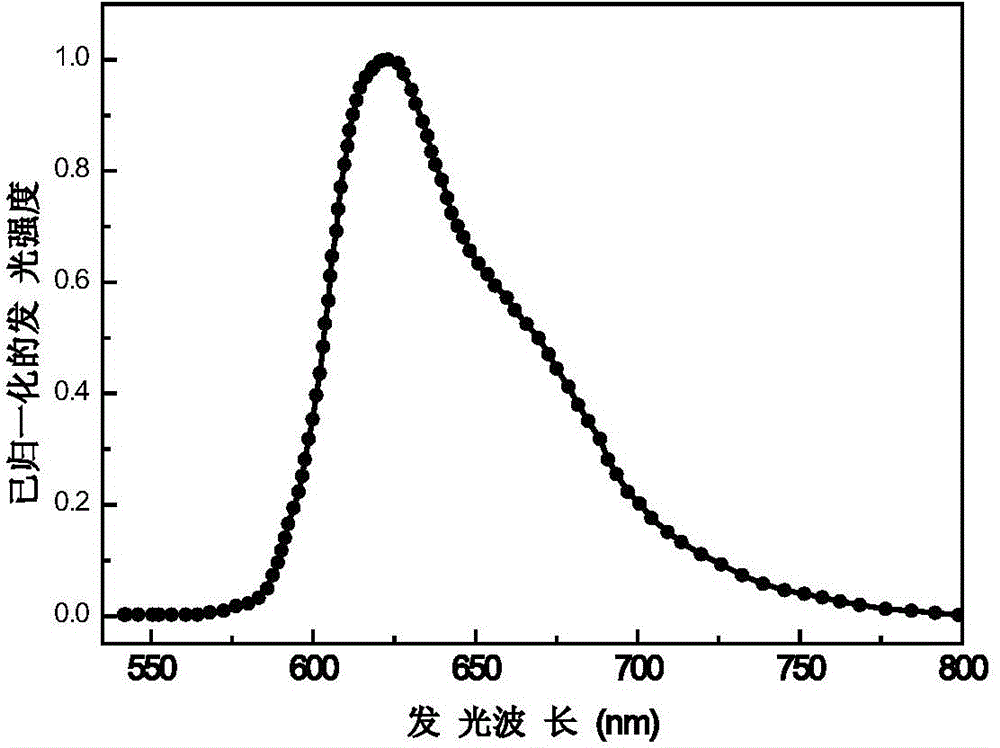
Embodiment 2
[0113] Bis[1-(9',9'-diphenylfluoren-2'-yl)isoquinoline-N,C3'](2,2,6,6-tetramethyl-3,5-heptanedione ) iridium organometallic iridium complex and preparation method thereof
[0114] (1) Synthesis of 2-pinacol borate-9,9-diphenylfluorene
[0115] Under an argon atmosphere, 4.69 mL of a 1.6 M n-butyllithium (7.5 mmol) n-hexane solution was added to a solution containing 2.38 g (6 mmol) of 2-bromo-9,9-diphenylfluorene and 35 mL of dry THF In a two-necked flask, the mixture was stirred at -78°C for 30 min. Add 1.53mL (1.40g, 7.5mmol) of isopropanol pinacol borate, and continue stirring for 3h after the reaction system naturally rises to room temperature. Add 20 mL of distilled water to terminate the reaction, extract 3 times with 40 mL of ether, and combine the organic phases. The organic phase was washed with saturated brine and dried over anhydrous magnesium sulfate. Filtrate, and rotary evaporate the solvent in the filtrate to obtain the crude product. The crude product was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com