Tropisetron hydrochloride injection and preparation method thereof
A technology for tropisetron hydrochloride and injection, applied in the field of medicine, can solve problems such as affecting the convenience of use, inconvenient use, general solubility, etc., and achieve the effects of easy operation, convenient and safe use, and high clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
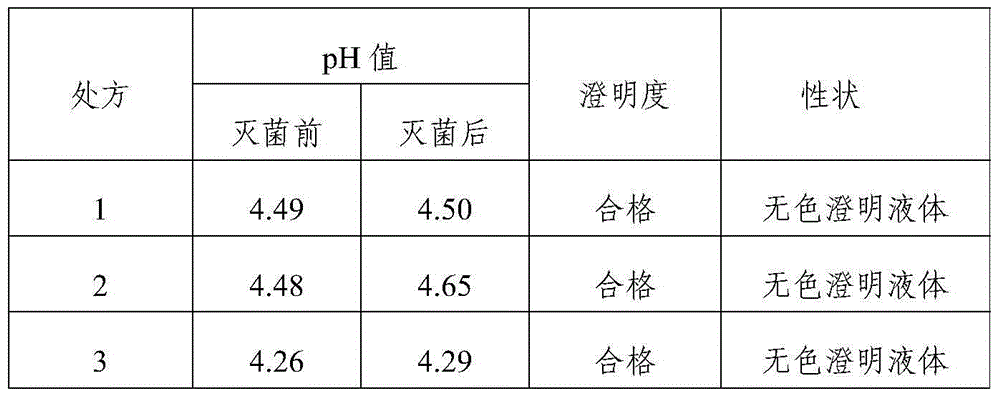
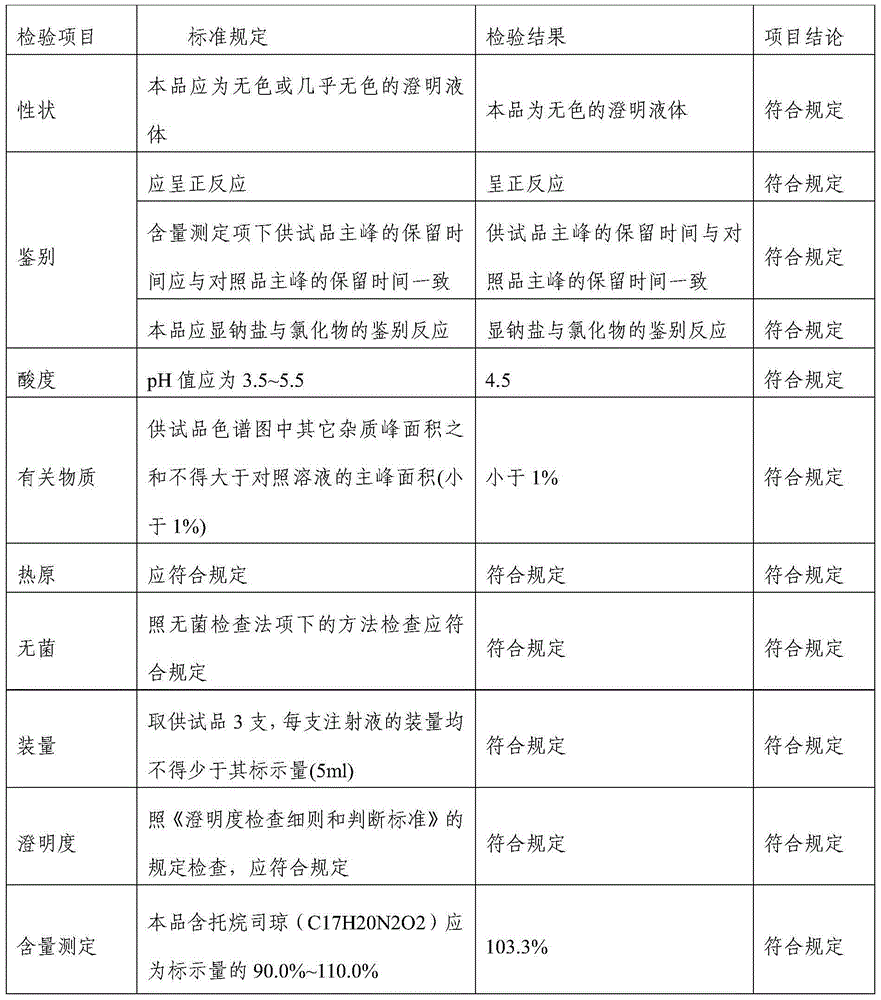
[0023] Tropisetron hydrochloride injection in this example comprises 5.64g tropisetron hydrochloride, 15g sodium acetate, 8.5g glacial acetic acid, 6.0g sodium chloride, and add water to 5000ml.
Embodiment 2-5
[0025] The difference between embodiment 2-5 and embodiment 1 is only that the consumption of each component is different, and concrete consumption is respectively as follows:
[0026] Component
[0027] Sodium acetate
Embodiment 6
[0029] Tropisetron hydrochloride injection in this embodiment comprises 1.13g tropisetron hydrochloride, 3g sodium acetate, 1.7g glacial acetic acid, 1.2g sodium chloride, and 3000ml water for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com