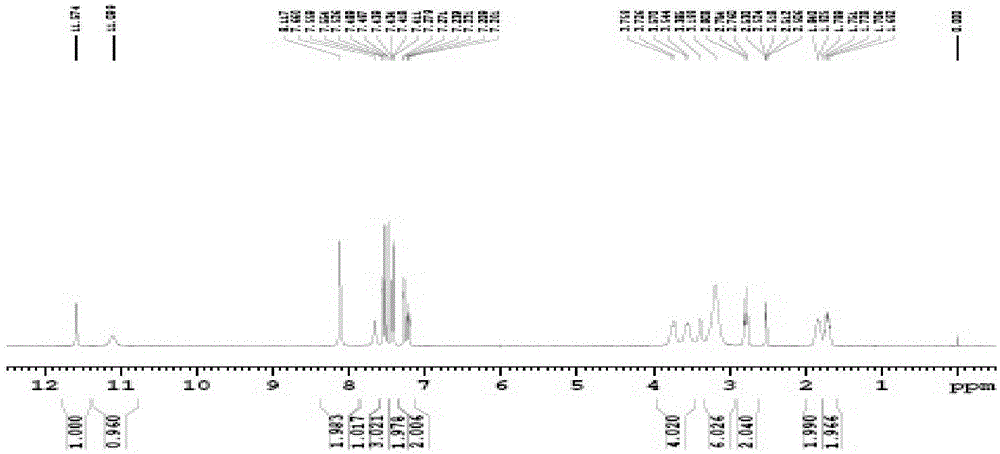
A kind of preparation method of vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-1h-5-cyanindole
A technology of -1H-5- and vilazodone, applied in the direction of organic chemistry, can solve the problems of difficult industrial large-scale production, low synthesis yield, high technical cost, etc., and achieve high synthesis efficiency, simple post-treatment operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation method one of 3-(4-chlorobutyl)-1H-5-cyanindole:
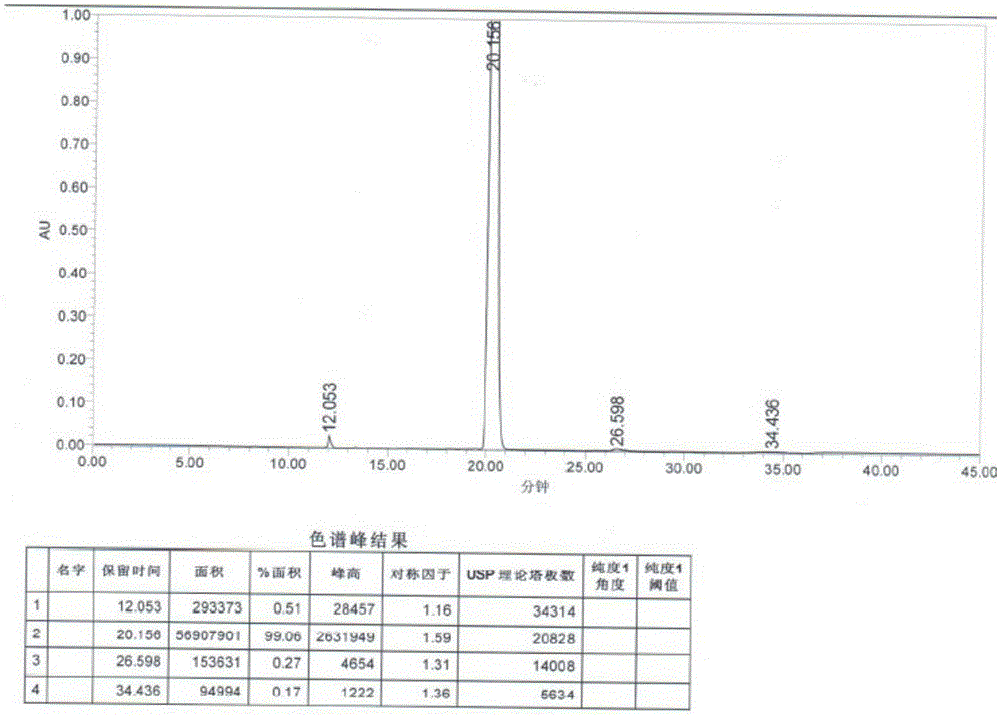
[0034] Disperse 18 grams of sodium borohydride in 200 mL of dichloromethane, slowly add 200 mL of dichloromethane solution dissolved with trichloroacetic acid (80 grams), and add 3-(4-chlorobutyryl)-1H-5- Cyanoindole (20 g), stirred at 30°C for 6 hours. The reaction solution was poured into water, and the water layer was separated. 1N aqueous sodium hydroxide solution was added to the organic phase to adjust the pH value to 7-9, the organic phase was washed with saturated aqueous sodium chloride solution, and the organic solvent was removed under reduced pressure. The crude product was recrystallized from methanol to obtain 15.5 g of off-white product. Yield is 82%, HPLC shows purity: 99.06%.
Embodiment 2
[0035] Embodiment 2: Preparation method two of 3-(4-chlorobutyl)-1H-5-cyanindole:
[0036] Disperse 18 grams of sodium borohydride in 600 mL of dichloromethane, slowly add 600 mL of dichloromethane solution dissolved with trichloroacetic acid (80 grams), and add 3-(4-chlorobutyryl)-1H-5- Cyanoindole (20 g), stirred at 30°C for 6 hours. The reaction solution was poured into water, and the water layer was separated. 1N aqueous sodium hydroxide solution was added to the organic phase to adjust the pH value to 7-9, the organic phase was washed with saturated aqueous sodium chloride solution, and the organic solvent was removed under reduced pressure. The crude product was recrystallized from methanol to obtain 16.8 g of off-white product with a yield of 88.9%.
Embodiment 3
[0037] Example 3: Preparation method three of 3-(4-chlorobutyl)-1H-5-cyanindole:
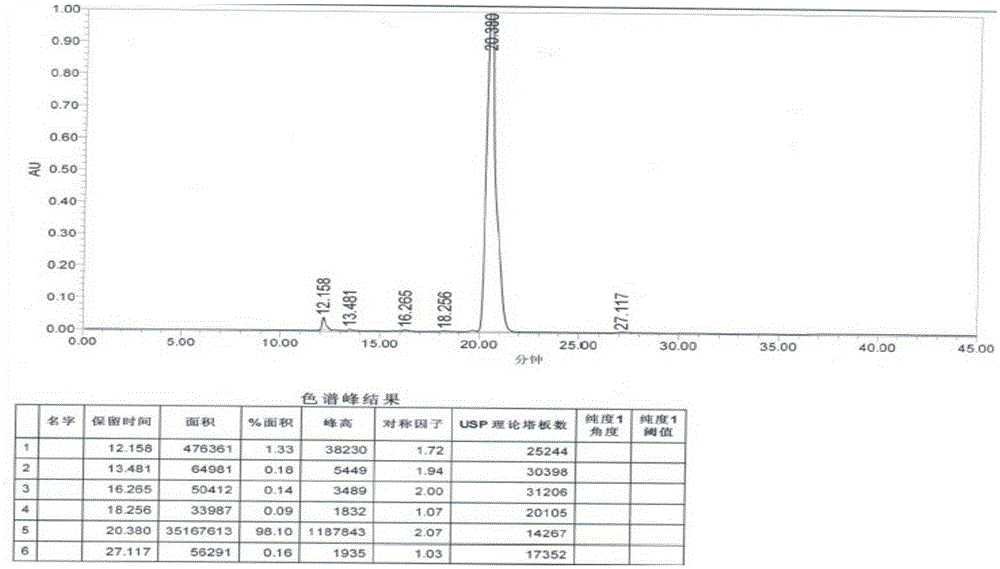
[0038] Disperse 18 grams of sodium borohydride in 400 mL of dichloromethane, slowly add 300 mL of dichloromethane solution dissolved with trichloroacetic acid (50 grams), and add 3-(4-chlorobutyryl)-1H-5- Cyanoindole (20 g), stirred at 30°C for 6 hours. The reaction solution was poured into water, and the water layer was separated. The organic phase was adjusted to pH 7-9 with 1N aqueous sodium hydroxide solution, washed with saturated aqueous sodium chloride solution, and the organic solvent was removed under reduced pressure. The crude product was recrystallized from methanol to obtain 16.1 g of off-white product. The yield was 85.2%, and the HPLC showed a purity of 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com