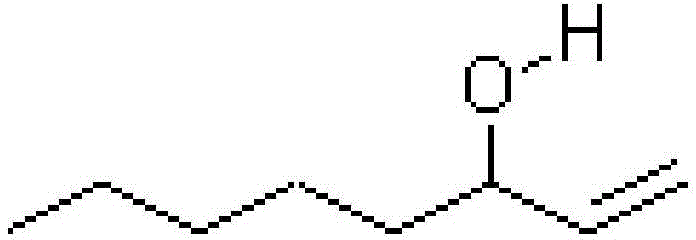
Synthesis method of 1-octylene-3-alcohol
A synthesis method and technology of octene, applied in the chemical field, can solve the problems of easy explosion, unsafe reaction conditions, and inapplicability, and achieve the effect of reducing fire accidents and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic method of 1-octen-3-alcohol comprises the following steps:
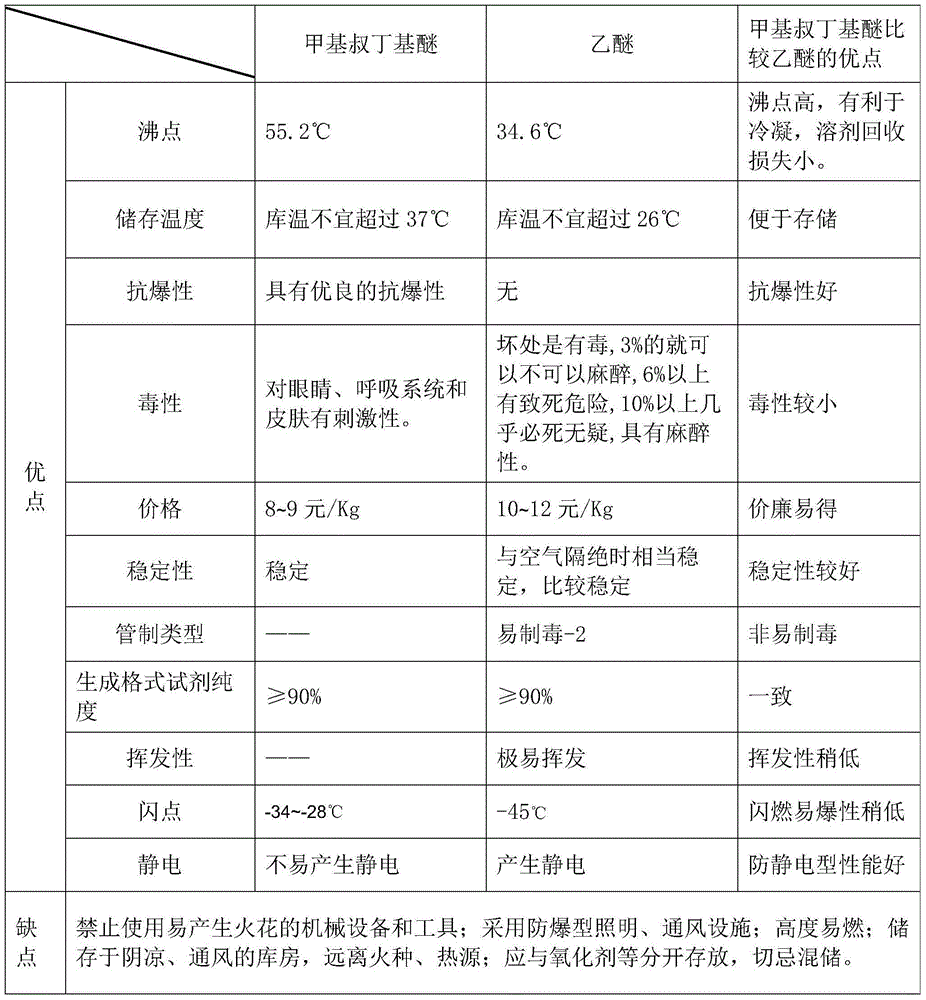
[0033] 1) Install a 1000mL reaction bottle equipped with a stirring device, with a dropping funnel on the top (the reaction system should be fully and strictly dried before the reaction), and a heating mantle on the bottom as a heating source. 280 g of anhydrous methyl tert-butyl ether and 16.5 g of magnesium granules (the purity of the magnesium granules is 99.5%) were first put into the reaction flask. Start stirring and warming up the system to the temperature of reflux, about 55°C, when there is a reflux phenomenon, stop stirring, add 50g of bromopentane and anhydrous methyl tert-butyl ether mixed solution (94g bromopentane+ Mix 55g of anhydrous methyl tert-butyl ether, molar ratio 1:1), bromopentane:magnesium particles=1:1.1, molar ratio, then start stirring, when the end of the cooler appears obvious reflux phenomenon, explain The reaction has already started. At this time, you should wait...
Embodiment 2
[0037] The synthetic method of 1-octen-3-alcohol comprises the following steps:
[0038] 1) Install a 1000mL reaction bottle equipped with a stirring device, with a dropping funnel on the top (the reaction system should be fully and strictly dried before the reaction), and a heating mantle on the bottom as a heating source. 280 g of anhydrous methyl tert-butyl ether and 16.5 g of magnesium granules (the purity of the magnesium granules is 98.5%) were first put into the reaction flask. Start stirring and warming up the system to the temperature of reflux, about 55°C, when there is a reflux phenomenon, stop stirring, add 50g of bromopentane and anhydrous methyl tert-butyl ether mixed solution (102g bromopentane+ Mix 60g of anhydrous methyl tert-butyl ether, the molar ratio is 1:1), bromopentane:magnesium grains=1:1, then start stirring, when the end of the cooler appears obvious reflux phenomenon, it means that the reaction has started At this time, you should wait quietly unti...
Embodiment 3
[0042] The synthetic method of 1-octen-3-alcohol comprises the following steps:
[0043]1) Install a 1000ml reaction bottle equipped with a stirring device, with a dropping funnel on the top (the reaction system should be fully and strictly dried before the reaction), and a heating mantle on the bottom as a heating source. 280 g of anhydrous methyl tert-butyl ether and 16.5 g of magnesium granules (the purity of the magnesium granules is 99.2%) were first put into the reaction flask. Start stirring and warming up the system to the temperature of reflux, about 55°C, when there is a reflux phenomenon, stop stirring, add 50g of bromopentane and anhydrous methyl tert-butyl ether mixed solution (98g bromopentane+ Mix 57g of anhydrous methyl tert-butyl ether, the molar ratio is 1:1), bromopentane:magnesium particles=1:1.05, then start stirring, and when there is obvious reflux phenomenon at the end of the cooler, it means that the reaction has started At this time, you should wait ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com