Bazedoxifene acetate capsule and preparation method thereof
A technology of bazedoxifene acetate, capsules, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
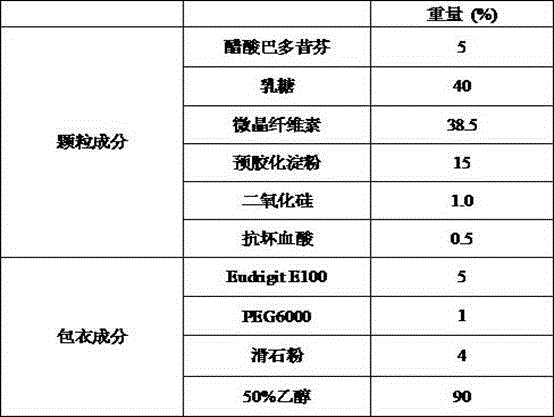
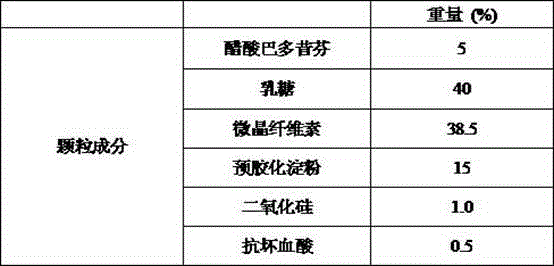
[0030] Example 1 Preparation of Bazedoxifene Acetate Capsules (20mg)
[0031]
[0032] Preparation Process:
[0033] (1) co-micronizing bazedoxifene acetate and silicon dioxide;
[0034] (2) Add and mix the raw materials of co-micronized powder with the prescribed amount of lactose, microcrystalline cellulose, pregelatinized starch, and ascorbic acid;
[0035] (3) Perform dry granulation of the above mixture to control the particle size so that the particles between 30-80 mesh account for 70-80%;
[0036] (4) Prepare a coating liquid, and use a fluidized bed to coat the granules;
[0037] (5) After the coating is completed, fill No. 1 capsules, with a loading range of 370-430 mg.
Embodiment 2
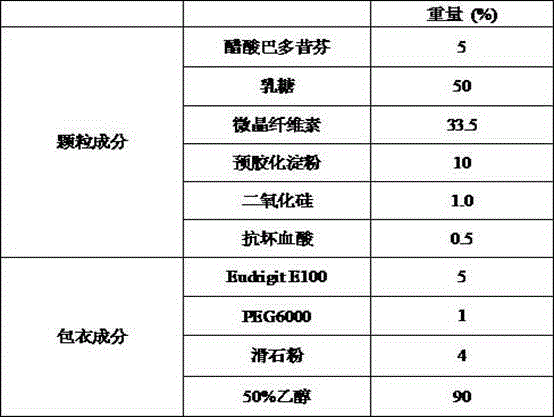
[0038] Preparation of Example 2 Bazedoxifene Acetate Capsules (20mg)
[0039]
[0040] Preparation Process:
[0041] (1) co-micronizing bazedoxifene acetate and silicon dioxide;
[0042] (2) Add and mix the raw materials of co-micronized powder with the prescribed amount of lactose, microcrystalline cellulose, pregelatinized starch, and ascorbic acid;
[0043] (3) Perform dry granulation of the above mixture to control the particle size so that the particles between 30-80 mesh account for 70-80%;
[0044] (4) Prepare a coating liquid, and use a fluidized bed to coat the granules;
[0045] (5) After the coating is completed, fill No. 1 capsules, with a loading range of 370-430 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com