Sofosbuvir film coating tablet preparation and preparation method thereof
A film coating, sofosbuvir technology, applied in the direction of antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effect of stable blood drug concentration and improved convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
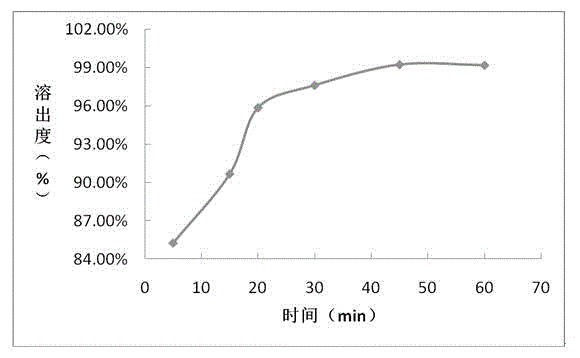
Embodiment 1
[0043] Prescription composition: Prescription amount (mg / tablet)
[0044] Sofosbuvir 400
[0045] Croscarmellose Sodium 96
[0046] Microcrystalline Cellulose 592
[0048] According to above-mentioned prescription quantity, preparation process is as follows:
[0049] (1) Material pretreatment: sieve sofosbuvir, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate for later use;
[0050] (2) Mixing: Weigh the active drug sofosbuvir, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate in the prescribed amount and mix evenly;
[0051] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0052] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0053] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0054] (6) Film coating: film-coat the qua...
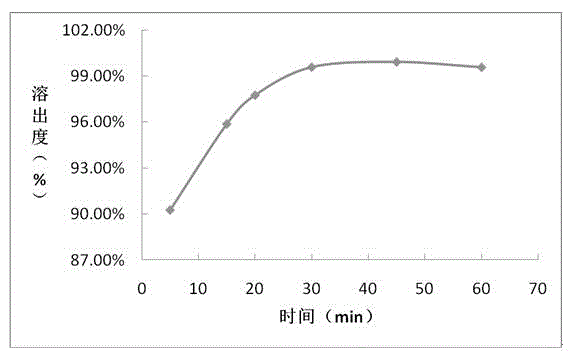
Embodiment 2
[0056] Prescription composition: Prescription amount (mg / tablet)
[0057] Sofosbuvir 400
[0058] Hypromellose 96
[0059] Microcrystalline Cellulose 592
[0061] According to above-mentioned prescription quantity, preparation process is as follows:
[0062] (1) Material pretreatment: sieve sofosbuvir, hypromellose, microcrystalline cellulose, and magnesium stearate for later use;
[0063] (2) Mixing: Weigh the prescription amount of active drug sofosbuvir, hypromellose, microcrystalline cellulose, and magnesium stearate and mix evenly;
[0064] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0065] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of hypromellose and magnesium stearate;
[0066] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0067] (6) Film coating: film-coat the qualified tablets prepared in (5).
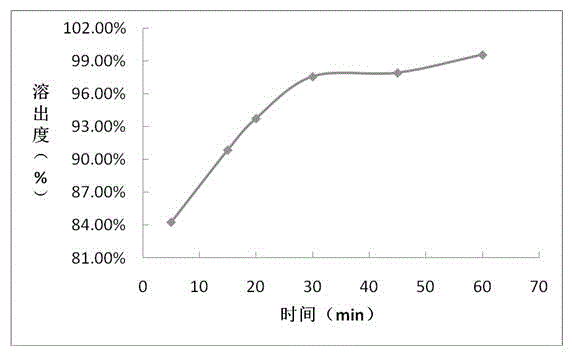
Embodiment 3
[0069] Prescription composition: Prescription amount (mg / tablet)
[0070] Sofosbuvir 400
[0071] Croscarmellose Sodium 96
[0072] Mannitol 592
[0074] According to above-mentioned prescription quantity, preparation process is as follows:
[0075] (1) Material pretreatment: sieve sofosbuvir, croscarmellose sodium, mannitol, and magnesium stearate for later use;
[0076] (2) Mixing: Weigh the active drug sofosbuvir, croscarmellose sodium, mannitol, and magnesium stearate in the prescribed amount and mix evenly;
[0077] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0078] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0079] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0080] (6) Film coating: film-coat the qualified tablets prepared in (5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com