Choline M receptor antagonist aclidinium bromide and preparation method thereof
A receptor antagonist, aclidinium bromide technology, applied to the choline M receptor antagonist aclidinium bromide and its preparation field, can solve the problems of high production cost, long reaction route and high production cost of aclidinium bromide , to achieve the effect of easy industrial production, short reaction route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
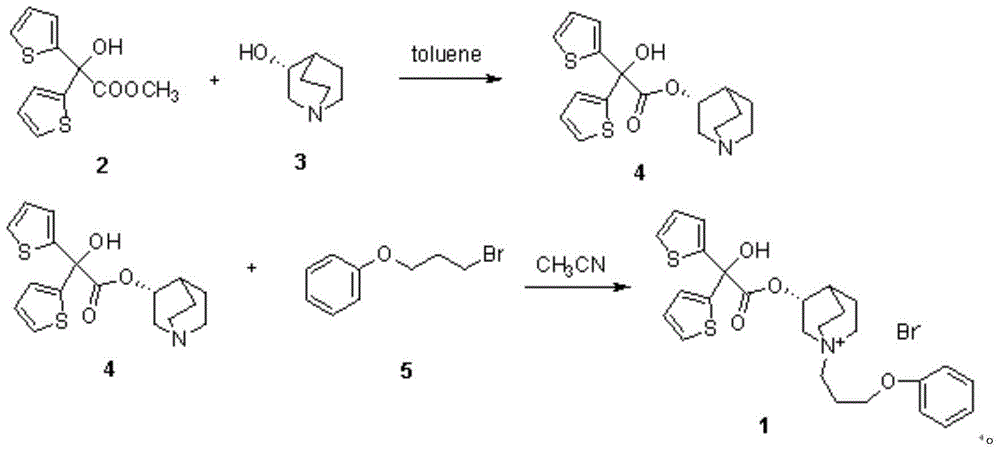
[0038] Take 63.5g (0.50mol) of 3-quinine alcohol, 60.5mL (0.75mol) of pyridine and 500mL of dichloromethane into a 1000mL round-bottomed flask, cool in an ice-water bath to 0-10°C, and add 67.1g of methyl oxalyl chloride dropwise (0.55mol), after dropping, heat, stir and reflux at 42°C for 8 hours. After the reaction is completed, add 200 mL of 2N hydrochloric acid to the reaction solution, stir for 10 minutes, separate the dichloromethane layer, and extract the water layer twice with dichloromethane. Dry with anhydrous sodium sulfate, wash with water to obtain an oily substance, add a hydrogen chloride-saturated ethanol solution to obtain 83.1 g of methyl oxalate hydrochloride as a white solid, yield 78.1%, mp: 162-164°C. 1 H NMR (400MHz, CDCl 3 )δ:1.90-2.25(m,5H),2.54(s,1H),3.36(brs,5H),3.84(s,1H),3.93(s,3H),12.48(s,1H); 13 C NMR (101MHz, CDCl 3 )δ: 157.24, 156.62, 77.44, 77.13, 76.81, 69.78, 53.91, 52.50, 46.36, 45.57, 24.04, 20.37, 17.01. ESI-MS: 240[M+H] + .
Embodiment 2
[0040] Take 500 mL of anhydrous tetrahydrofuran, 48.9 g (0.30 mol) of 2-bromothiophene, and 7.2 g (0.3 mol) of magnesium powder, first add a small amount of 2-bromothiophene to the reaction solution, and then add 2 grains of iodine to initiate the reaction. Then add 2-bromothiophene dropwise, drop it in 30 minutes, continue stirring at room temperature for 2 hours, add 37.4 g (0.15 mol) of methyl oxalate 3-quinine alcohol ester hydrochloride in batches under stirring, dropwise, and stir at room temperature React for 30 minutes, then heat, stir and reflux at 60°C for 6 hours. After the reaction is complete, add 200 mL of saturated ammonium chloride solution, stir for 10 minutes, extract with ethyl acetate, wash the ethyl acetate layer with water, and dry the ethyl acetate layer with anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 30.2 g of white color, yield 57.7%, mp: 149-151°C. 1 H-NMR(400MHz,DMSO)δ:1.24(m,2H),1.54-1.58(m,3H),1.91(m,1H...
Embodiment 3
[0042] Take 63.5g (0.50mol) of R-3-quinine alcohol, 60.5mL (0.75mol) of pyridine and 500mL of dichloromethane into a 1000mL round bottom flask, cool in an ice-water bath to 0-10°C, and add methyl oxalyl chloride dropwise 67.1g (0.55mol), after dropping, heat, stir and reflux at 45°C for 8 hours. After the reaction is completed, add 200mL of 2N hydrochloric acid to the reaction solution, stir for 10 minutes, separate the dichloromethane layer, and extract the water layer twice with dichloromethane. The methane layer was dried with anhydrous sodium sulfate, washed with water to obtain an oily substance, and an ethanol solution saturated with hydrogen chloride was added to obtain a white solid methyl-R-3-quinine oxalate, yield 68.5%, mp: 176-178°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com