Capsicine-camptothecin anti-cancer drug conjugate and preparation method and application thereof
An anticancer drug, capsaicin technology, applied in the directions of drug combinations, antitumor drugs, pharmaceutical formulations, etc., to achieve the effects of high yield, good safety, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
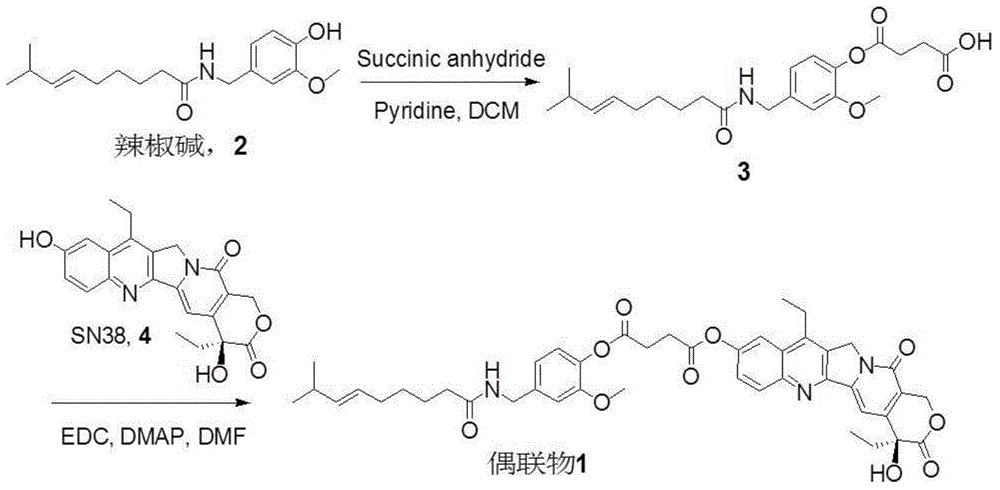
[0039] The preparation of embodiment 1 capsaicin-SN-38 conjugate (C10 hydroxyl)
[0040] The preparation method of a kind of capsaicin-SN-38 conjugate of this embodiment (synthetic route sees figure 1 ), including the following steps:
[0041] (1) Synthesis of capsaicin derivative 3
[0042] Dissolve capsaicin (658mg, 2mmol) and DMAP (289mg, 2.4mmol) in 1ml of pyridine, add succinic anhydride (517.4mg, 5mmol), react at 40°C for 4h, remove the reaction solvent; the residual solid is dissolved in dichloromethane , and then washed with 5% citric acid and saturated brine respectively; the organic phase was dried over anhydrous sodium sulfate, and after filtration, the filtrate was collected and the solvent was removed under reduced pressure; the solid was separated and purified by column chromatography (dichloromethane:methanol=100 : 1) obtain capsaicin derivative 3 after.
[0043] capsaicin derivative 3 1 The H NMR nuclear magnetic data is as follows:
[0044] 1 H NMR (400M...
Embodiment 2
[0048] Embodiment 2 Preparation of capsaicin-SN-38 conjugate self-emulsifying particles
[0049] The conjugate 1 (20 mg) of Example 1 was dissolved in 1 mL Tween 80 solution to obtain a self-emulsifying preparation, and then the self-emulsifying preparation was slowly injected into water (final concentration 2 mg / mL), and gently shaken and vibrated to form nanoparticles.
[0050] Take a small amount of the above-prepared nanoparticle solution, spot it on a copper grid, negatively stain it with 2% uranyl acetate, and observe it with a transmission film. Depend on figure 2 It can be seen that the self-emulsifying preparation forms milk particles of relatively uniform size after being diluted with water.
Embodiment 3
[0051] Example 3 In vitro cytotoxicity evaluation
[0052] In order to evaluate the killing ability of the conjugate 1 self-emulsified particles obtained in Example 2 on tumor cells, taking intestinal cancer cells HCT-116 and SW480, lung cancer cells H1299 and breast cancer cells MDA-MB-231 and MCF-7 as examples, The drug efficacy was evaluated by MTT method, and the IC of the drug on different cells was investigated. 50 Value (drug concentration at the time of inducing tumor cell apoptosis by 50%), with CPT-11, capsaicin and SN-38 as controls. The evaluation results are shown in Table 1.
[0053] Table 1 In vitro cytotoxicity evaluation results of each test drug
[0054]
[0055] As can be seen from Table 1, after 48 hours of co-cultivation of the self-emulsifying particles of the conjugate 1 and the cells, the results showed that the IC of the conjugate 1 on SW480 cells 50 0.07±0.03μM, its in vitro anti-tumor activity is 408 times that of CPT-11, 3 times that of SN-38;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tumor volume | aaaaa | aaaaa |
| Tumor volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com