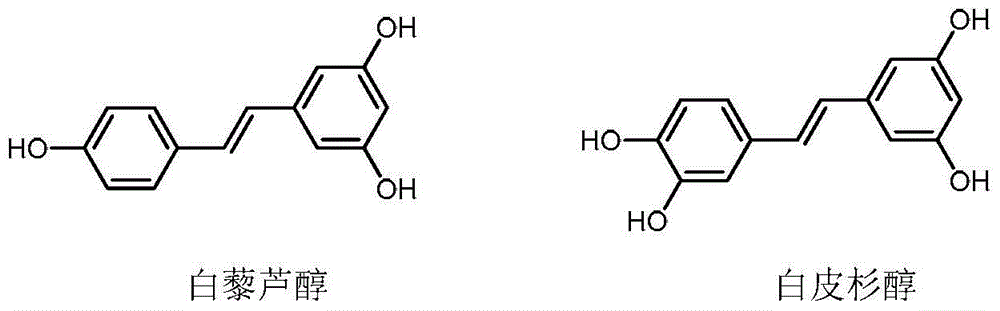
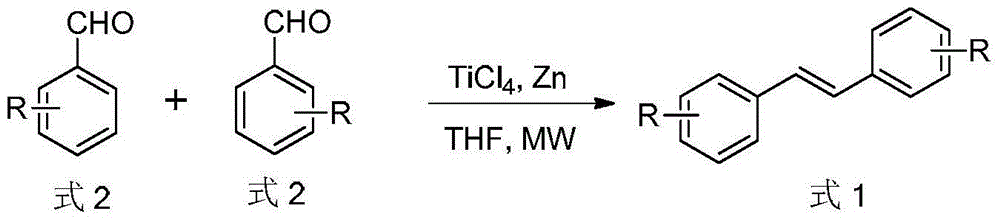
Method for synthesizing trans-stilbene compound under assistance of microwave
A technology for stilbene and microwave synthesis, applied in the field of chemical industry, can solve the problems of expensive metal catalysts, low atom economy, harsh reaction conditions, etc., and achieve the effects of short reaction time, convenient post-processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
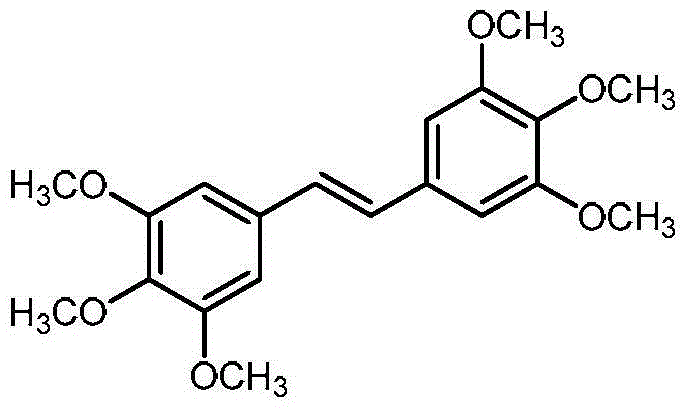
[0022] The preparation of 3,3',4,4',5,5'-hexamethoxytrans-stilbene comprises the following steps:
[0023] Titanium tetrachloride (1.09 ml, 10 mmol) was added dropwise to a mixture of zinc powder (1.30 g, 20 mmol) and tetrahydrofuran (20 ml) under ice-cooling. After the dropwise addition, 3,4,5-trimethoxybenzaldehyde (1.96 g, 10 mmol) was added to the above mixture, and stirred at room temperature for 1 hour. The above reaction system was refluxed in a microwave reactor for 10 minutes (65°C). TLC was used to detect the progress of the reaction. After the reaction was completed, it was filtered. A large amount of dilute hydrochloric acid was added to the filtrate to precipitate a solid, which was filtered by suction and dried. The solid was recrystallized with chloroform-ethanol to obtain White solid 3,3',4,4',5,5'-hexamethoxytrans-stilbene 1.53g, yield 85%.
[0024] The characterization data of the product are: 1 H NMR (400MHz, DMSO-d 6 )δ:ppm 7.15(s,2H),6.89(s,4H),3.82(s,1...
Embodiment 2
[0027] The preparation of 4,4'-dihydroxy-3,3',5,5'-tetramethoxytrans-stilbene comprises the following steps:
[0028] Titanium tetrachloride (1.32ml, 15mmol) was added dropwise to a mixture of zinc powder (1.95g, 30mmol) and tetrahydrofuran (20ml) under ice-cooling. After the dropwise addition, 4′-hydroxy-3,5,-dimethoxybenzaldehyde (1.82 g, 10 mmol) was added to the above mixture, and stirred at room temperature for 1 hour. The above reaction system was refluxed in a microwave reactor for 10 minutes (65°C). TLC was used to detect the progress of the reaction. After the reaction was completed, it was filtered. A large amount of dilute hydrochloric acid was added to the filtrate to precipitate a solid, which was filtered by suction and dried. The solid was recrystallized with chloroform-ethanol to obtain White solid 4,4'-dihydroxy-3,3',5,5'-tetramethoxytrans-stilbene 1.36g, yield 82%.
[0029] The characterization data of the product are: 1 H NMR (400MHz, DMSO-d 6 )δ:ppm 8.45...
Embodiment 3
[0032] The preparation of 3,3',5,5'-tetramethoxy trans-stilbene comprises the following steps:
[0033] Titanium tetrachloride (1.31 ml, 12 mmol) was added dropwise to a mixture of zinc powder (3.90 g, 60 mmol) and tetrahydrofuran (20 ml) under an ice bath. After the dropwise addition, 3,5-dimethoxybenzaldehyde (1.66 g, 10 mmol) was added to the above mixture, and stirred at room temperature for 1 hour. The above reaction system was refluxed in a microwave reactor for 15 minutes (65°C), and the progress of the reaction was detected by TLC. After the reaction was completed, it was filtered, and a large amount of dilute hydrochloric acid was added to the filtrate to precipitate a solid, which was filtered by suction and dried. The solid was recrystallized with chloroform-ethanol to obtain White solid 3,3',5,5'-tetramethoxytrans-stilbene 1.31g, yield 87%.
[0034] The characterization data of the product are: 1 H NMR (400MHz, DMSO-d 6 )δ:ppm 7.20(s,2H),6.77(d,J=2.0Hz,4H),6.40(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com