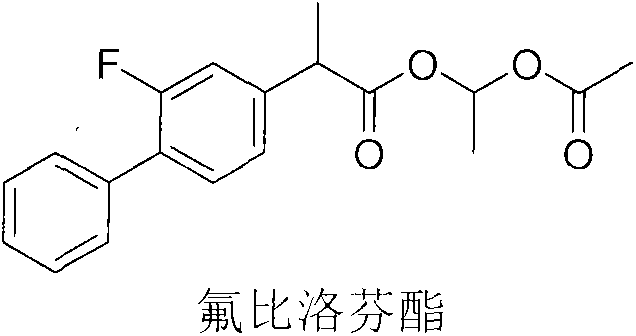
Flurbiprofen axetil pharmaceutical composition for relieving fever
A technology of flurbiprofen axetil and composition, which is applied in the field of flurbiprofen axetil pharmaceutical composition, and can solve problems such as the application of flurbiprofen axetil that has not been reported in literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
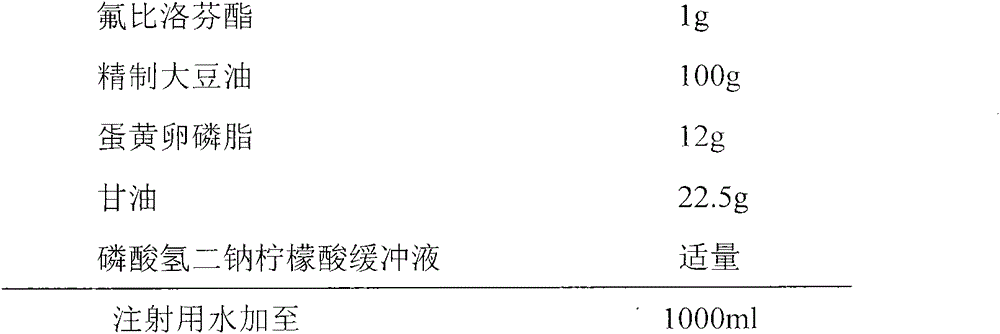
[0022] prescription:
[0023]
[0024] Process:
[0025] (1) Preparation of water phase: add glycerin to water to dissolve, heat to 70°C, and set aside;
[0026] (2) Preparation of oil phase: heating soybean oil to 70°C, adding egg yolk lecithin and flurbiprofen axetil, stirring to dissolve;
[0027] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 70°C, high-speed shear dispersion, shear speed of 10,000 rpm, and time of 15 minutes, to form colostrum;
[0028] (4) pH value adjustment: quickly cool down the colostrum in step (3) below 30°C, and adjust the pH value to 4.5-6.5 with disodium hydrogen phosphate citric acid buffer solution (the molar ratio of disodium hydrogen phosphate to citric acid is 4:1) ;
[0029] (5) High-pressure homogenization: the colostrum in step (4) is homogenized under high pressure by a microfluidizer for 3 times, the pressure is 800-1200 bar, and the temperature is controlled belo...
Embodiment 2
[0033] prescription:
[0034]
[0035] Process:
[0036] (1) Preparation of water phase: add glycerin to water to dissolve, heat to 70°C, and set aside;
[0037] (2) Preparation of oil phase: heat refined soybean oil to 70°C, add egg yolk lecithin and flurbiprofen axetil, stir to dissolve;
[0038] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 70°C, high-speed shear dispersion, shear speed of 10,000 rpm, and time of 15 minutes, to form colostrum;
[0039] (4) pH value adjustment: quickly cool down the colostrum in step (3) below 30°C, and adjust the pH value to 4.5-6.5 with disodium hydrogen phosphate citric acid buffer solution (the molar ratio of disodium hydrogen phosphate to citric acid is 4:1) ;
[0040] (5) High-pressure homogenization: the colostrum in step (4) is homogenized under high pressure by a microfluidizer for 3 times, the pressure is 800-1200 bar, and the temperature is controlled below ...
Embodiment 3
[0044] Effects on Rat Fever Induced by Dry Yeast
[0045] Take Wistar rats with normal body temperature, weighing 180-220g, pre-adapt to the environment for 5 days, measure the rectal temperature once a day, and measure the rectal temperature every 1 hour before the experiment, for 3 consecutive times, take the average value as the normal value, for The same rat can be used as a fever model if the temperature difference between two consecutive times does not exceed 0.3°C. 60 qualified rats were randomly divided into 6 groups, 10 in each group. The rats in each group were subcutaneously injected with 20% fresh dry yeast in normal saline suspension at 10ml / kg. After 6 hours, the blank group was given normal saline. Positive Control group gives lysine 0.4g / kg, and experimental group gives respectively flurbiprofen axetil 10mg / kg (embodiment 1 sample), flurbiprofen axetil 20mg / kg (embodiment 1 sample), flurbiprofen axetil Ester 10mg / kg (embodiment 2 sample), flurbiprofen axetil 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com