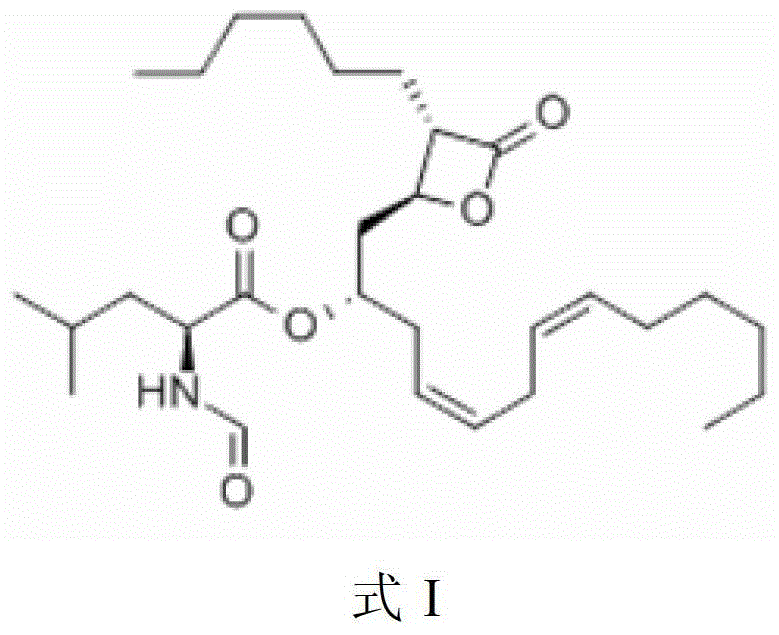
Purification method of liprestatin
A technology of liprestatin and a purification method, which is applied in the field of riprestatin purification, can solve the problems of unmentioned yield, content, large energy consumption, high cost, etc., achieve reduction of production cost, increase production efficiency, overcome The effect of high equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Preparation of butylamine acetate: add 30g of acetic acid into 36.5g of butylamine under stirring, set aside.
[0093] 10L of liprestatin fermented liquid obtained by fermenting Streptomyces toxins was adjusted to pH 3.0 with dilute sulfuric acid, 1.5L of ethanol and 3.60L of butyl acetate were added, stirred and extracted, left to stand and separated to obtain the upper organic solvent layer 4.1 L, washed with water; concentrated to dryness, recovered butyl acetate, and obtained 665.2 g of dark yellow paste. Add 1.8 L of isooctane for extraction, filter, add 0.70 L of acetonitrile, stir for 10-15 min, and let stand to separate layers; remove the lower layer, concentrate to dryness, recover acetonitrile, and obtain 96.4 g of a yellow oily paste.
[0094] Add 0.50 L of isooctane and 65 g of butylamine acetate, stir to dissolve, and filter; add 0.40 L of isooctane to the filtrate, gradually cool down to -18°C, and crystallize to obtain light yellow riprestatin crystals. ...
Embodiment 2
[0096] Preparation of butylamine acetate: add 60g of acetic acid into 73g of butylamine under stirring, set aside.
[0097] 10L of riprestatin fermented liquid obtained by fermenting Streptomyces toxins was adjusted to pH 4.10 with dilute sulfuric acid, 3.0L of ethanol and 6.0L of butyl acetate were added, stirred and extracted, left to stand and separated to obtain the upper organic solvent layer 8.2 L, washed with water; concentrated to dryness, recovered butyl acetate, and obtained 689.4 g of dark yellow paste. Add 2.4L of isooctane to the mixture for extraction, filter, add 1.44L of acetonitrile, stir for 10-15min, and let stand to separate layers; remove the lower layer, concentrate to dryness, recover acetonitrile, and obtain 100.3g of a yellow oily paste.
[0098] Add 0.38 L of isooctane and 38 g of butylamine acetate, stir to dissolve, and filter; add 0.30 L of isooctane to the filtrate, gradually cool down to -18°C, and crystallize to obtain light yellow riprestatin c...
Embodiment 3
[0100] Preparation of butylamine acetate: add 60g of acetic acid into 73g of butylamine under stirring, set aside.
[0101] 10L of riprestatin fermented liquid obtained by fermenting Streptomyces toxins was adjusted to pH 5.0 with dilute phosphoric acid, 1.0L of ethanol and 3.0L of butyl acetate were added, stirred and extracted, left to stand and separated to obtain the upper organic solvent layer 3.2 L, washed with water; concentrated to dryness, recovered butyl acetate, and obtained 622.8 g of dark yellow paste. Add 1.5 L of isooctane to the mixture for extraction, filter, add 0.8 L of acetonitrile, stir for 10-15 min, and let stand to separate layers; remove the lower layer, concentrate to dryness, recover acetonitrile, and obtain 87.2 g of a yellow oily paste.
[0102] Add 0.60 L of isooctane and 75 g of butylamine acetate, stir to dissolve, and filter; add 0.36 L of isooctane to the filtrate, gradually cool down to -18°C, and crystallize to obtain light yellow riprestati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com