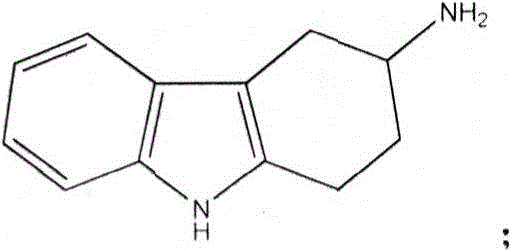
A kind of resolution method of 3-amino-1,2,3,4-tetrahydrocarbazole
A technology of tetrahydrocarbazole and amino group, applied in the field of medicine and chemical industry, can solve the problems of low resolution yield, high price, high price of mandelic acid, etc., and achieve the effect of reducing production cost and saving raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
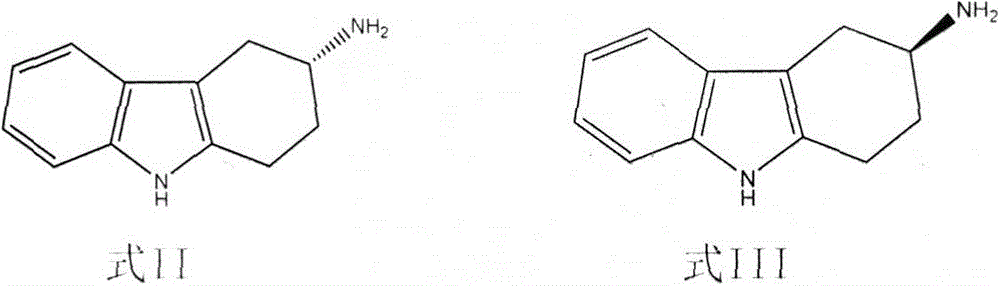
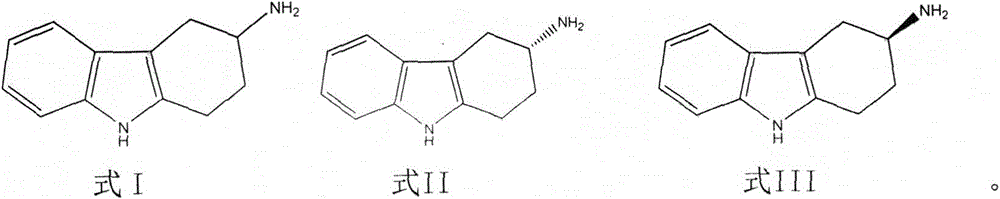
[0048] Add 500L of water, 250L of methanol, and 29Kg of acetic acid into a 1000L reactor, add 100Kg of 3-amino-1,2,3,4-tetrahydrocarbazole, heat up to 45-50°C and stir to dissolve, add 6Kg of activated carbon, and stir Decolorize for 30min and filter. Add 44Kg of L-tartaric acid to the filtrate, heat up to 50-55°C, stir to dissolve, then slowly cool down to 30-32°C, and filter. The filter cake is enriched in S-3-amino-1,2,3,4-tetrahydrocarbazole. The filtrate is enriched with R-3-amino-1,2,3,4-tetrahydrocarbazole for later use.
[0049] The filter cake enriched with S-3-amino-1,2,3,4-tetrahydrocarbazole was recrystallized again with 500 L of water and 180 L of methanol to obtain a single enantiomer. The resolution yield is 35%. Add this salt to 15 times the weight of water, heat up to 75°C, stir to dissolve, adjust the pH value to above 12 with 30% sodium hydroxide aqueous solution, and precipitate white powder, cool down to below 40°C, filter, and wash with appropriate amo...
Embodiment 2
[0051] Add the filtrate enriched in R-3-amino-1,2,3,4-tetrahydrocarbazole in Example 1 into a 2000L reaction kettle, start stirring, add 375 liters of methanol, 3Kg of activated carbon, and heat up to 40~ Stir at 45°C for 30min and filter. Adjust the temperature of the filtrate to 45-48°C, add 28Kg of L-tartaric acid as a resolving agent, stir to dissolve, slowly cool down to 20-25°C, and filter. The filter cake is enriched with R-3-amino-1,2,3,4-tetrahydrocarbazole, put the filter cake into a 2000L reaction kettle, add 500L water, 700L methanol, 6Kg activated carbon, stir and raise the temperature to about 65°C and keep After 30 minutes, cool down to 50°C and filter, slowly cool down to 28-30°C, and filter. The filter cake is a salt of single enantiomer R-3-amino-1,2,3,4-tetrahydrocarbazole and L-tartaric acid, and the resolution yield is 42%. Add this salt to 30 times the weight of water, heat up to 65°C, stir to dissolve, adjust the pH value to above 12 with 30% potassium...
Embodiment 3
[0053] Add 500L of water, 100L of methanol, and 31Kg of acetic acid into a 1000L reactor, add 100Kg of 3-amino-1,2,3,4-tetrahydrocarbazole, heat up to 45-50°C and stir to dissolve, add 6Kg of activated carbon, and stir Decolorize for 30min and filter. Add 40.3Kg of D-tartaric acid to the filtrate, heat up to 50-55°C, stir to dissolve, then slowly cool down to 30-32°C, and filter. The filter cake is enriched in R-3-amino-1,2,3,4-tetrahydrocarbazole. The filtrate is enriched with S-3-amino-1,2,3,4-tetrahydrocarbazole for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com