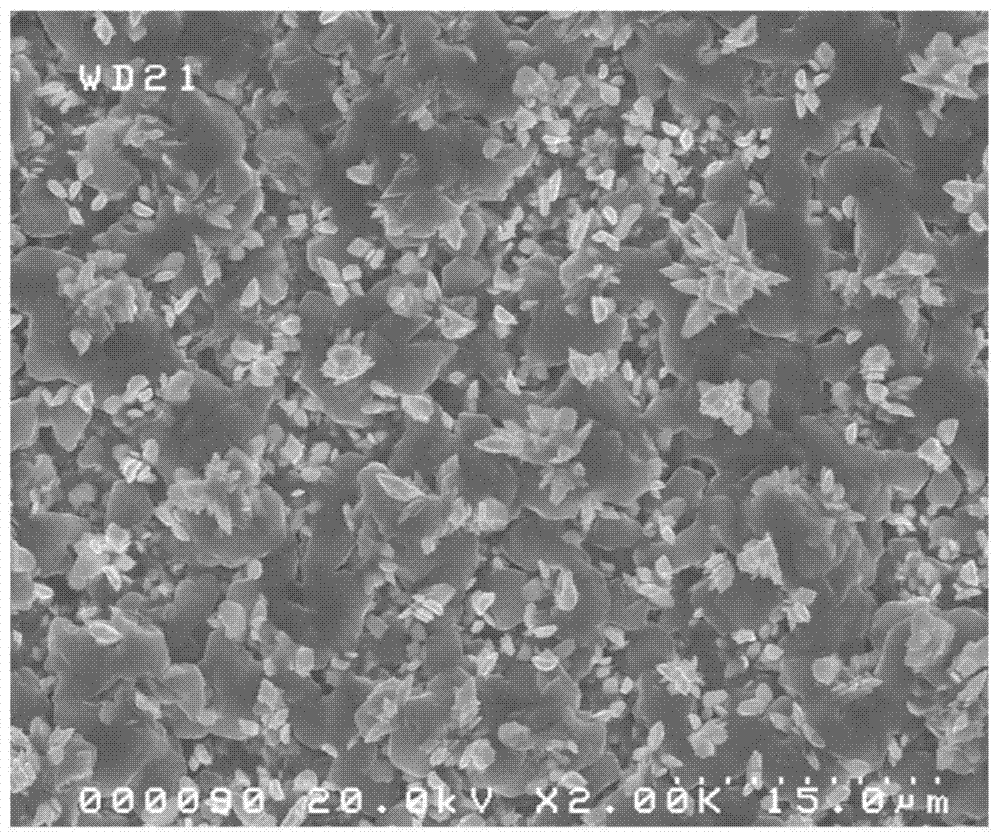
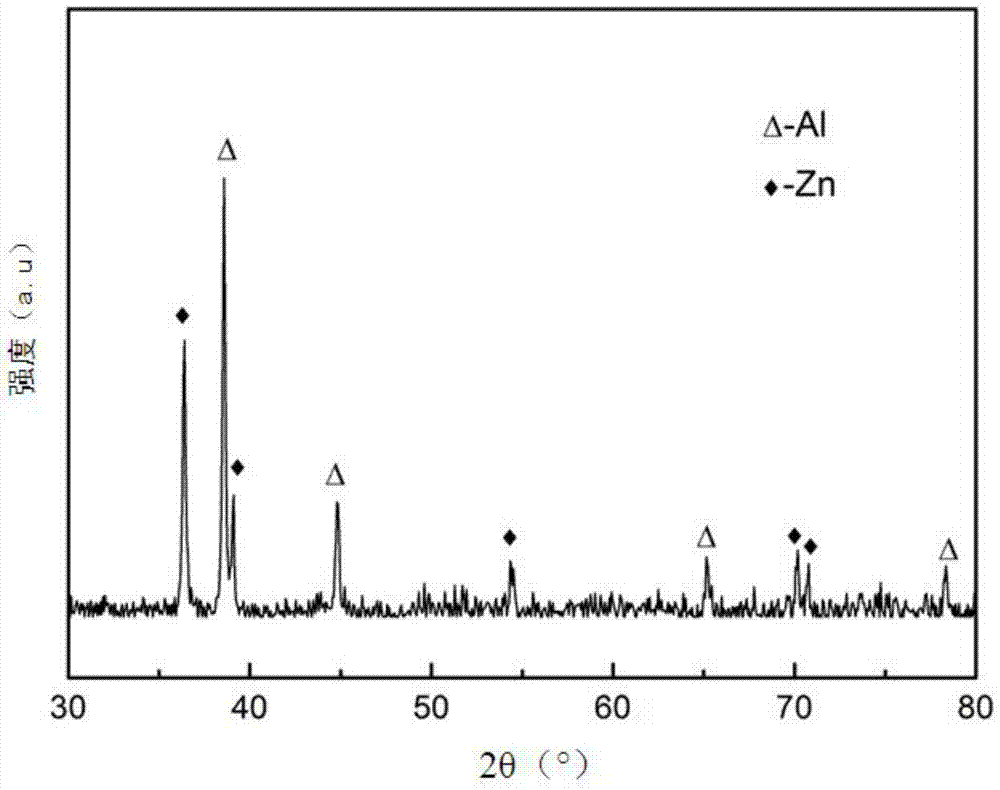
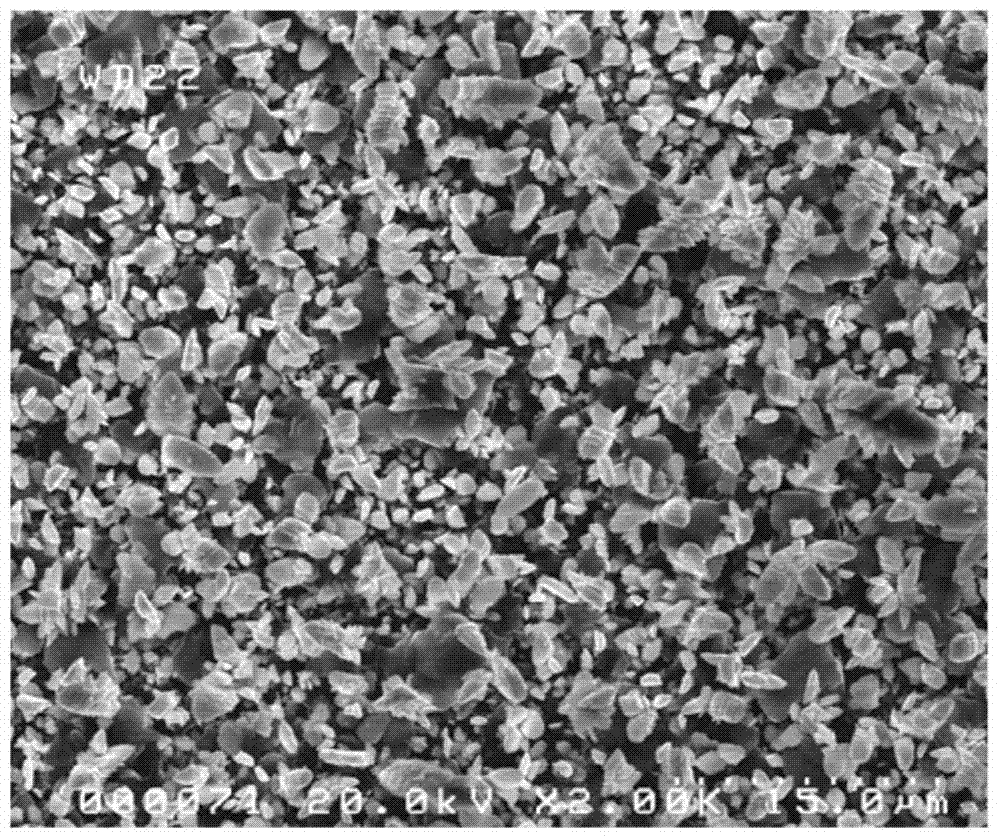
Method for co-depositing al-zn alloy coating in ionic liquid system
An ionic liquid and alloy plating technology, applied in the direction of cells, electrolysis process, electrolysis components, etc., can solve the problems of poor plating thickness and composition uniformity, serious equipment corrosion, high energy consumption, etc. The effect of small environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The method for co-depositing an Al-Zn alloy coating in an ionic liquid system of the present invention comprises the following steps:
[0032] (1) Preparation of disubstituted imidazolium chloride-aluminum chloride type ionic liquid
[0033] A small amount of anhydrous aluminum chloride is added to the disubstituted imidazole chloride several times to form a disubstituted imidazole chloride-aluminum chloride ionic liquid at room temperature; the disubstituted imidazole chloride and AlCl 3The molar ratio is 1:2; the disubstituted imidazole chloride is 1-methyl-3 ethyl imidazole chloride.
[0034] (2) Preparation of disubstituted imidazolium chloride-zinc chloride type ionic liquid
[0035] Anhydrous zinc chloride is added to disubstituted imidazole chloride and heated to 80°C to form a disubstituted imidazole chloride-zinc chloride ionic liquid; the disubstituted imidazole chloride and ZnCl 2 The molar ratio is 1:2; the disubstituted imidazole chloride is 1-methyl-3 et...
Embodiment 2
[0049] The method for co-depositing an Al-Zn alloy coating in an ionic liquid system of the present invention comprises the following steps:
[0050] (1) Preparation of disubstituted imidazolium chloride-aluminum chloride type ionic liquid
[0051] A small amount of anhydrous aluminum chloride is added to the disubstituted imidazole chloride several times to form a disubstituted imidazole chloride-aluminum chloride ionic liquid at room temperature; the disubstituted imidazole chloride and AlCl 3 The molar ratio is 1:4; the disubstituted imidazole chloride is 1-ethyl-3 methyl imidazole chloride.
[0052] (2) Preparation of disubstituted imidazolium chloride-zinc chloride type ionic liquid
[0053] Anhydrous zinc chloride is added to disubstituted imidazole chloride and heated to 60°C to form a disubstituted imidazole chloride-zinc chloride ionic liquid; the disubstituted imidazole chloride and ZnCl 2 The molar ratio is 1:4; the disubstituted imidazole chloride is 1-ethyl-3 me...
Embodiment 3
[0067] The method for co-depositing an Al-Zn alloy coating in an ionic liquid system of the present invention comprises the following steps:
[0068] (1) Preparation of disubstituted imidazolium chloride-aluminum chloride type ionic liquid
[0069] A small amount of anhydrous aluminum chloride is added to the disubstituted imidazole chloride several times to form a disubstituted imidazole chloride-aluminum chloride ionic liquid at room temperature; the disubstituted imidazole chloride and AlCl 3 The molar ratio is 1:1; the disubstituted imidazole chloride is 1-ethyl-3 methyl imidazole chloride.
[0070] (2) Preparation of disubstituted imidazolium chloride-zinc chloride type ionic liquid
[0071] Anhydrous zinc chloride is added to disubstituted imidazole chloride and heated to 80°C to form a disubstituted imidazole chloride-zinc chloride ionic liquid; the disubstituted imidazole chloride and ZnCl 2 The molar ratio is 1:1; the disubstituted imidazole chloride is 1-ethyl-3 me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com