Application of peptidylarginine deiminase 2 (PAD2) to preparation of reagent for clinical diagnosis of tumors
A technology of peptidyl arginine deiminase and diagnostic reagents, which is applied in the field of application of peptidyl arginine deiminase 2 in the preparation of tumor clinical diagnostic reagents, and can solve problems such as distribution of different tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 PAD2 as a detection example of tumor blood markers
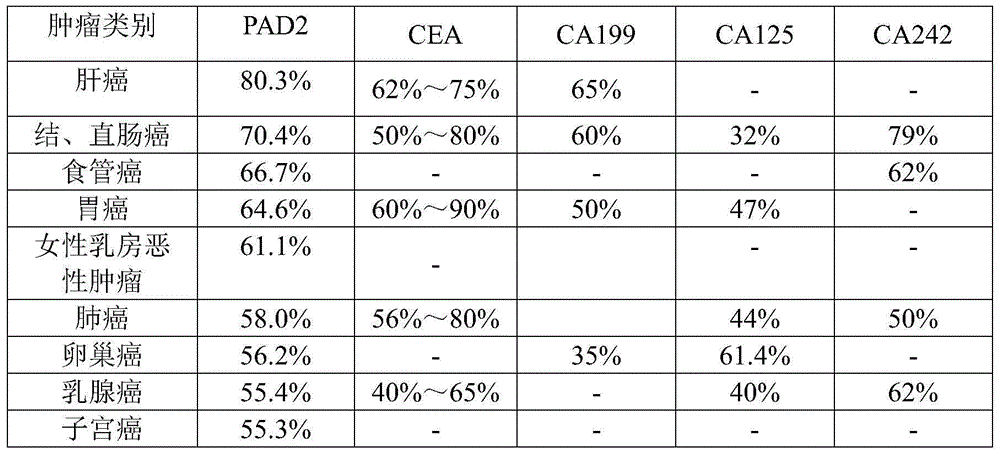
[0054] PAD2 was used as a tumor blood marker to detect the blood of tumor patients. The number of tumor patients and the types of tumors are shown in Table 1.
[0055] The detection method is:
[0056] (1) Dilute the blood sample to be tested 10 times with carbonate buffer (pH 9.6), add it to a blank microtiter plate, and incubate at 37°C for 2 hours;
[0057] (2) discard the liquid in the hole, and wash the plate three times with PBST washing solution (the PBST washing solution refers to the PBS solution plus Tween-20, the pH of the PBS solution is 7.4, and the concentration of Tween-20 is 0.1%, percent by volume);
[0058] (3) Add 200 μL / well of blocking solution (0.5% BSA, mass percent) to each well, and incubate at 37°C for 1 hour;
[0059] (4) Discard the liquid in the well, and wash the plate three times with PBST washing solution;
[0060] (5) Add 100 μL / well of PAD2 antibody dilution (1:4000 dilut...
Embodiment 2 PAD2 test example 2
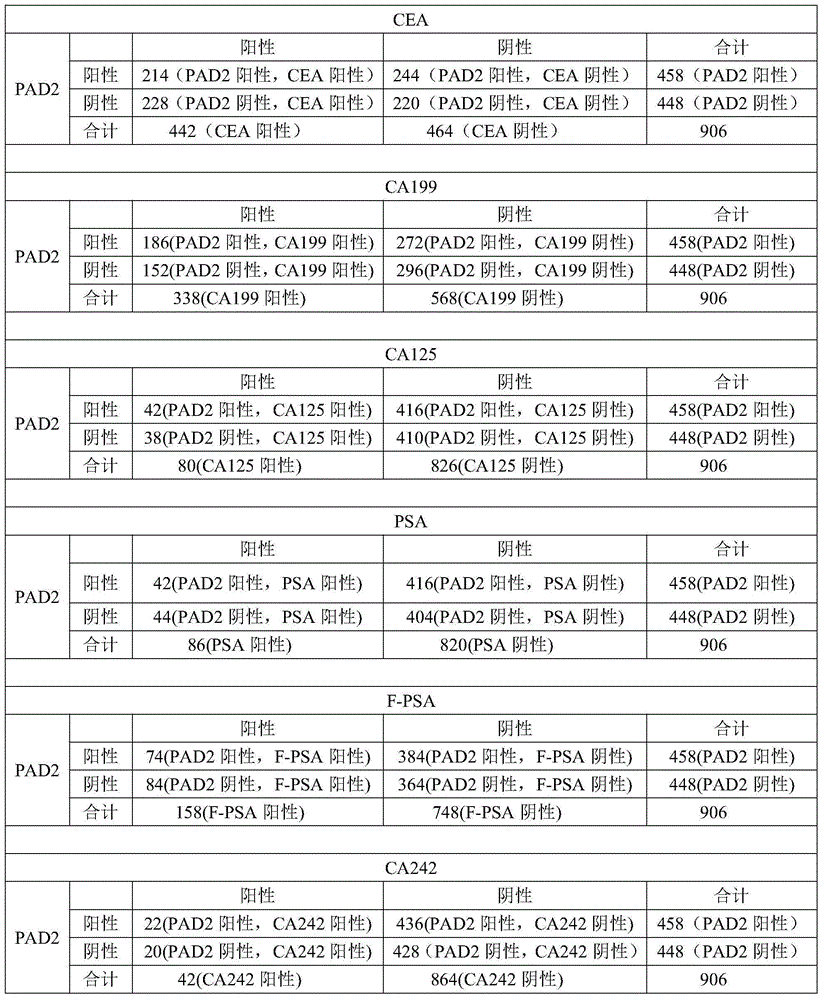
[0074] Using PAD2 as a tumor blood marker, the patient's blood was detected, and the detection method was the same as in Example 1. At the same time, with CEA, CA199, F / PSA, CA125, PSA, CA242 in the prior art as tumor blood markers, the blood of tumor patients is detected (the detection method is a conventional method in the prior art; these prior art The blood sample detection data of the tumor blood markers in is the patient outpatient data of a certain hospital, and this data can be used as one of the basis for diagnosing whether the patient has a tumor; the present invention also uses the blood samples of some patients to detect PAD2). The positive rate of each tumor blood marker was compared, and the cross-comparison between PAD2 and each tumor marker.
[0075] Results: The number of patients (a total of 906 cases) and the number of positive cases are shown in Table 3. It can be seen from Table 3 that PAD2 can be used as a tumor blood marker with good specificity.
[00...
Embodiment 3 PAD2 test example 3
[0079] Using PAD2 as a blood marker, the blood of patients with hepatitis B, general inflammation (increased leukocytes, neutrophils, and decreased lymphocyte ratio), kidney disease (including nephrotic syndrome, renal failure, and nephritis) was detected. See Table 4 for details of the number and types of diseases.
[0080] The detection method is the same as in Example 1.
[0081] Result: The number of positive cases and the positive rate are shown in Table 4. As can be seen from Table 4, PAD2, as a blood marker, can also be used to detect hepatitis B, common inflammation (with leukocytes, neutrophils, symptoms of decreased lymphocyte ratio) ), kidney disease (including nephrotic syndrome, renal failure, nephritis) and other diseases, the test results can be used as one of the basis for clinical diagnosis.
[0082] Table 4
[0083] disease category
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com