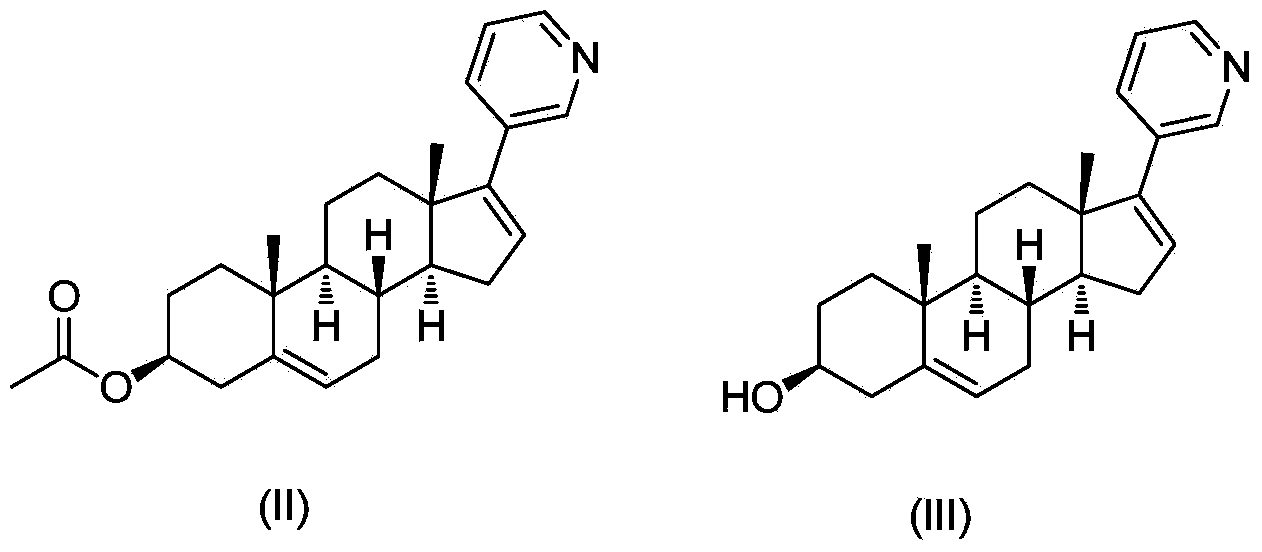
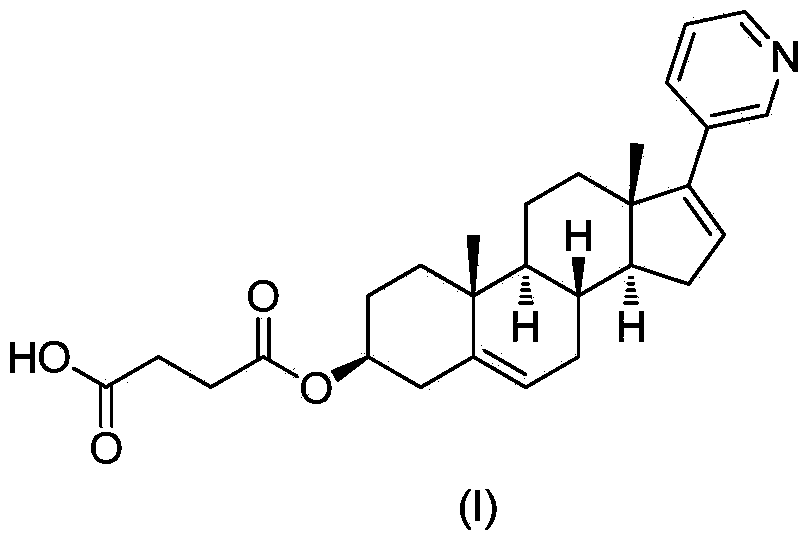
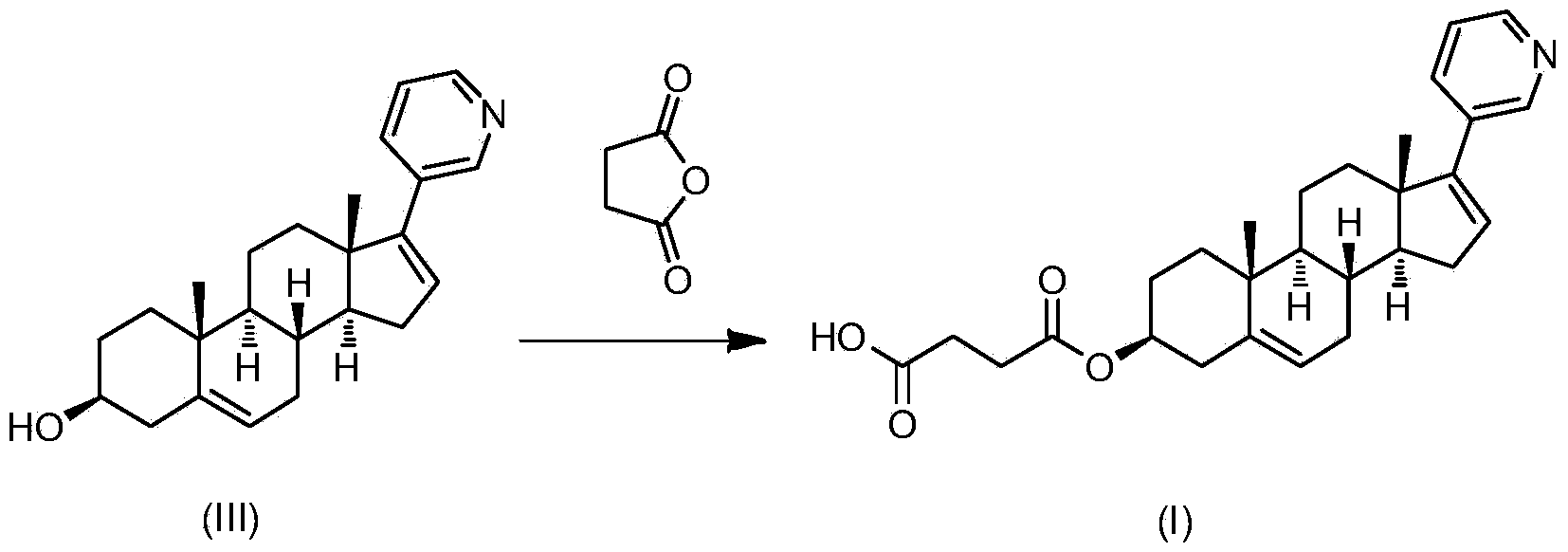
Abiraterone single succinic acid ester and preparation method thereof
A technology of abiraterone and succinate, which is applied in the field of drug synthesis, can solve problems such as safety discount of abiraterone acetate, and achieve the effect of simple post-processing and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Abiraterone (3.50g, 10mmol), succinic anhydride (1.20g, 12mmol) were sequentially added in tetrahydrofuran (25mL), and then pyridine (3.95g, 50mmol); the reaction mixture was stirred at 65-75°C for 20 hours, TLC (thin-layer chromatography plate) shows that abiraterone has reacted completely (developing agent is sherwood oil: the volume ratio of ethyl acetate=1: 3), and reaction mixture is added sherwood oil, after filtering, filter cake is washed with methanol and Abiraterone monosuccinate (3.03 g, yield 67.3%, white powder) was obtained by drying.
[0024] Proton NMR spectrum (500MHz, DMSO) δH12.16-12.18(s, 1H), 8.56(d, 1H), 8.40-8.41(d-d, 1H), 7.72-7.74(t-t, 1H), 7.29-7.32(m , 1H), 6.09(m, 1H), 5.36(d, 1H), 4.40-4.50(m, 1H), 2.44(s, 4H), 2.25-2.27(d, 2H), 2.16-2.21(m, 1H ), 1.99-2.05(m, 3H), 1.45-1.83(m, 8H), 1.36-1.44(m, 1H), 0.96-1.02(d, 8H);
[0025] Carbon NMR spectrum (500MHz, DMSO) δC19.4, 22.2, 22.5, 28.4, 29.1, 29.5, 31.7, 32.5, 33.8, 37.4, 37.5, 37.6, 38.5,...
Embodiment 2
[0029] Abiraterone (3.50g, 10mmol), succinic anhydride (1.20g, 12mmol) were successively added in ethyl acetate (200mL), then triethylamine (2.0g, 20mmol) was added; the reaction mixture was stirred at 65-70°C After 48 hours, TLC (thin layer chromatography plate) showed that abiraterone had reacted completely (developing solvent is sherwood oil: ethyl acetate=1: 3), and the reaction mixture was added sherwood oil, after filtering, the filter cake was washed with methanol And dried to obtain abiraterone monosuccinate (3.38g, yield 75.1%, white powder). The product was subjected to H NMR spectroscopy ( 1 H-NMR), carbon nuclear magnetic resonance spectrum ( 13 C-NMR) and liquid chromatography-mass spectrometry (LCMS) confirmed the structure as the target compound abiraterone monosuccinate.
Embodiment 3
[0031] Abiraterone (3.50g, 10mmol), succinic anhydride (1.20g, 12mmol) were added successively in toluene (50mL), and then 4-(N,N-dimethylamino)pyridine (0.61g, 5mmol) was added for reaction mixing The solution was stirred at 115-120°C for 8 hours, TLC (thin-layer chromatography plate) showed that the abiraterone had completely reacted (the polarity of the developer was petroleum ether: ethyl acetate=1:3), and petroleum ether was added to the reaction mixture. , after filtration, the filter cake was washed with methanol and dried to obtain abiraterone monosuccinate (3.82g, yield 85.1%, white powder). The product was subjected to H NMR spectroscopy ( 1 H-NMR), carbon nuclear magnetic resonance spectrum ( 13 C-NMR) and liquid chromatography-mass spectrometry (LCMS) confirmed the structure as the target compound abiraterone monosuccinate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com