Chiral cinchona alkaloid-squaramide catalyst (CSF-MSNs) loaded on inorganic meso-porous silicon and preparation thereof
A technology of chiral square amide and cinchona base, which is applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc., and can solve problems such as high price, difficult product separation, and pollution , to achieve strong activity, high thermodynamic stability, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
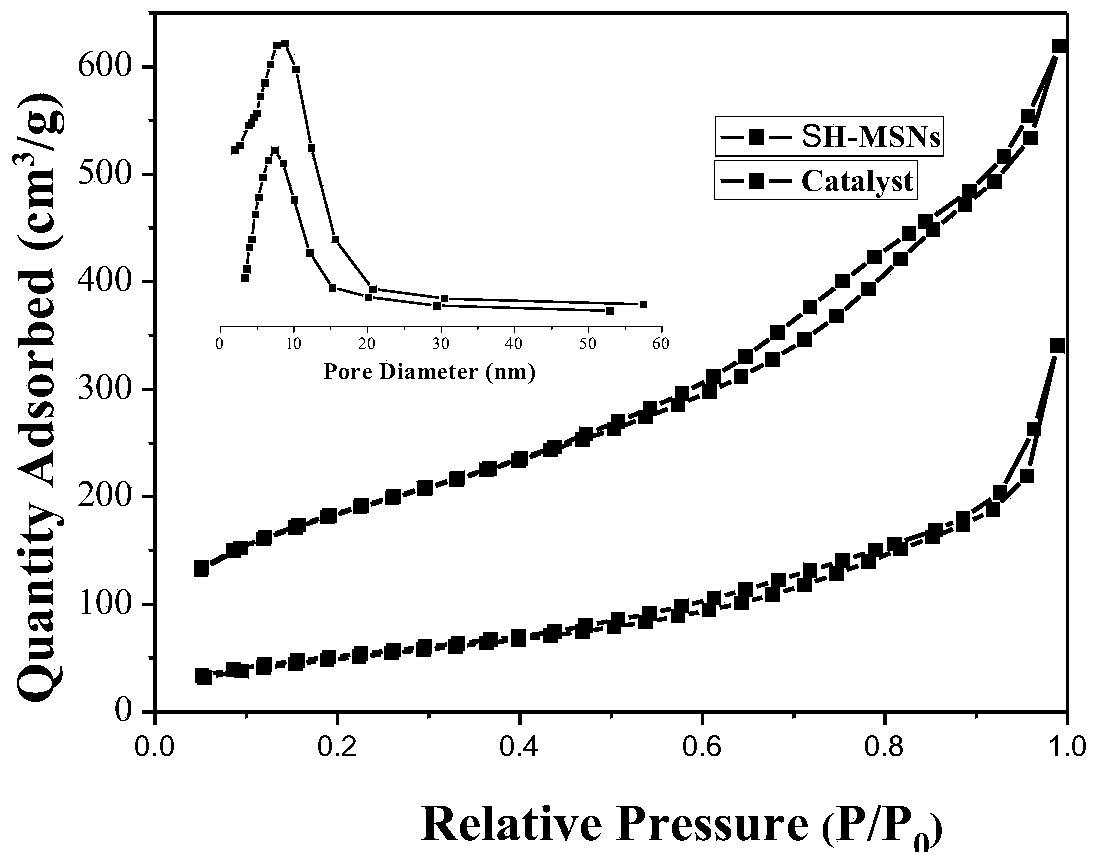
Image
Examples
Embodiment 1
[0026] (1) Synthesis of thiol-functionalized carrier SH-MSNs
[0027] Get a 200mL three-necked flask, add 1.92g of cetrimonium tosylate (CTATos), 0.35g of triethanolamine (TEAH 3 ) and 100mL deionized water, mechanically stirred at 80°C for 1h;
[0028] Quickly add 14.58g of tetraethyl orthosilicate (TEOS), then in the process of stirring, dropwise add 1.19g of γ-mercaptopropyltrimethoxysilane (MPTMS) with a disposable dropper, and control the dropping within 5 minutes , the reaction continued to be mechanically stirred at a speed of 1200r / min at 80°C for 2h;
[0029] After the reaction, filter with suction, wash, and then dry in a vacuum oven at 100° C. for 20 h. Then it was ground and extracted in alcoholic hydrochloric acid solution and aqueous hydrochloric acid solution at 80°C for 24 hours respectively to obtain white powdery solid SH-MSNs.
[0030] (2) Synthesis of chiral square amide homogeneous catalyst:
[0031] In a 10mL round bottom flask, add 127mg (0.36mmol) o...
Embodiment 2
[0039] (1) Synthesis of thiol-functionalized carrier SH-MSNs
[0040] Take a 250mL three-necked flask, add 3.84g of cetrimonium p-toluenesulfonate (CTATos), 0.70g of triethanolamine (TEAH3) and 200mL of deionized water, and mechanically stir at 80°C for 1h;
[0041] Quickly add 29.16g of tetraethyl orthosilicate (TEOS), and then add 2.38g of γ-mercaptopropyltrimethoxysilane (MPTMS) dropwise with a disposable dropper during stirring, and control the dropping within 5 minutes , the reaction continued to be mechanically stirred at a speed of 1200r / min at 80°C for 2h;
[0042]After the reaction, filter with suction, wash, and then dry in a vacuum oven at 100° C. for 20 h. Then it was ground and extracted in alcoholic hydrochloric acid solution and aqueous hydrochloric acid solution at 80°C for 24 hours respectively to obtain white powdery solid SH-MSNs.
[0043] (2) Synthesis of chiral square amide homogeneous catalyst:
[0044] In a 10mL round bottom flask, add 127mg (0.36mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com