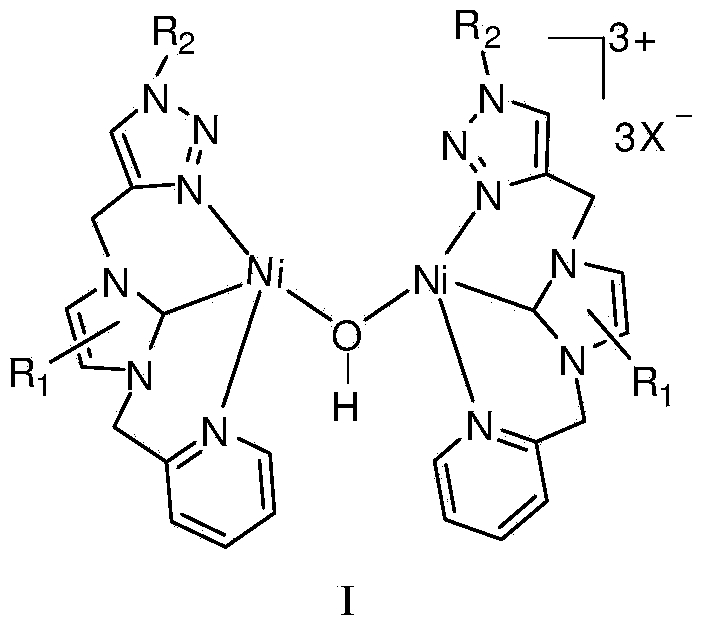
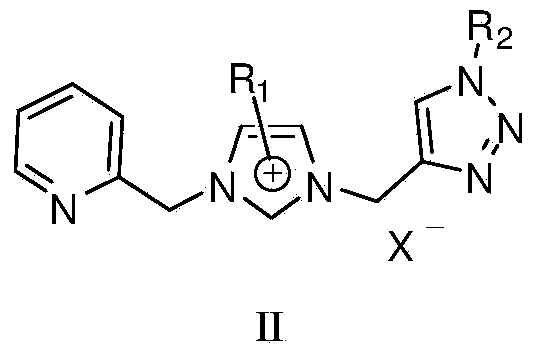
1,2,3-triazole functionalized N-heterocyclic carbene binuclear nickel compound and preparation method thereof
A nitrogen-heterocyclic carbene and nickel compound technology, applied in the field of metal organic chemistry, can solve the problem of less research on dual-nuclear nickel compounds, and achieve the effects of novel structure, easy functionalization, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L1 (477 mg, 1 mmol), silver oxide (116 mg, 0.5 mmol) and 20 mL of acetonitrile into a Schlenk reaction tube for 10 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 6 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum-dried at 30°C for 10h to obtain the molecular structure: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 1 was 700 mg, and the yield was 55%. 1 H NMR (400MHz, d 6 -DMSO): δ8.54(b, 1H), 8.30(s, 1H), 7.89(t, J=7.2Hz, 1H), 7.79(s, 2H), 7.48(d, J=7.6Hz, 1H) ,7.39-7.33(m,6H),5.64(s,2H),5.68(s,4H)ppm. 13 C NMR (400MHz, d 6 -DMSO): δ156.9 (Ni-C), 153.9, 150.1, 141.2, 138.0, 137.5, 136.2, 129.3, 128.8, 125.1, 124.2, 123.9, 123.1, 123.0, 53.6, 53.5, 44.2ppm.
[0044] Utilizi...
Embodiment 2
[0048]
[0049] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L2 (527mg, 1mmol), silver oxide (116mg, 0.5mmol) and 20mL of acetonitrile into the Schlenk reaction tube, react for 15 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 7 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum dried at 30°C for 10h to obtain the molecular structure formula: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 2 was 714 mg, and the yield was 52%. 1 H NMR (400MHz, d 6 -DMSO): δ8.79(b,1H),8.46-8.44(m,1H),8.39(s,1H),8.29(t,J=8.0Hz,1H),8.18-8.16(m,1H), 8.06(d,J=8.0Hz,1H),7.78-7.72(m,3H),7.37-7.30(m,6H),5.98(s,2H),5.68(s,2H),5.63(s,2H) ppm. 13 C NMR (400MHz, d 6 -DMSO): δ157.9 (Ni-C), 153.9, 149.9, 147.6, 143.1, 141.1, 140.5, 136.2, 131.7, 130.1, 129.3, 128.8, 1...
Embodiment 3
[0054]
[0055] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L3 (443mg, 1mmol), silver oxide (116mg, 0.5mmol) and 20mL of acetonitrile into the Schlenk reaction tube, react for 15 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 8 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum dried at 30°C for 10h to obtain the molecular structure formula: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 3 was 747 mg, and the yield was 62%. 1 H NMR (400MHz, DMSO-d 6 ):δ9.05(s,1H,triazole),8.63(d,1H),8.16-8.14(m,2H),7.87(s,1H),7.82(s,1H),7.61-7.58(m,1H ),5.68(s,2H),5.65(s,2H),4.18(t,J=7.8Hz,2H),1.79-1.74(m,2H),1.31-1.24(m,2H),0.90(t, 3H,J=7.2Hz,). 13 C NMR (100MHz, DMSO-d 6 ): δ154.2 (Ni-C), 149.5, 148.7, 142.0, 136.8, 125.1, 123.2, 123.1, 122.5, 114.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com