Method for synthesizing polysubstituted olefins
A multi-substituted, olefin technology, applied in the preparation of halogenated hydrocarbons, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of high cost, pollute the environment, difficult to apply to large-scale production, etc., to improve activity and durability, The effect of low catalyst dosage and recyclable catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Catalyst Preparation
[0023] (1) Weigh 465.9 mg (5 mmol) of aniline and dissolve it in 50 ml of 1M hydrochloric acid aqueous solution to obtain solution 1;
[0024] (2) Weigh 22 mg (0.125 mmol) of palladium chloride and dissolve it in 50 ml of 1M hydrochloric acid aqueous solution to obtain solution 2;
[0025] (3) Solution 1 and Solution 2 were mixed, stirred for 2 minutes, and then left to stand for 24 hours. Then adjust the pH to 7.0 with 1M aqueous sodium hydroxide solution to produce a flocculent precipitate;
[0026] (4) Centrifuge and wash the precipitate with deionized water. Then add 6 ml of deionized water and shake the suspension. The suspension can be stored in the refrigerator (4 degrees) for a long time;
[0027] (5) Take 0.6 ml of the above suspension and dilute to 5 ml with deionized. 0.5 ml of it was centrifuged to remove water, and the precipitate was used to catalyze the Suzuki coupling reaction (1 mmol reaction scale). IPC analysis proves t...
Embodiment 2
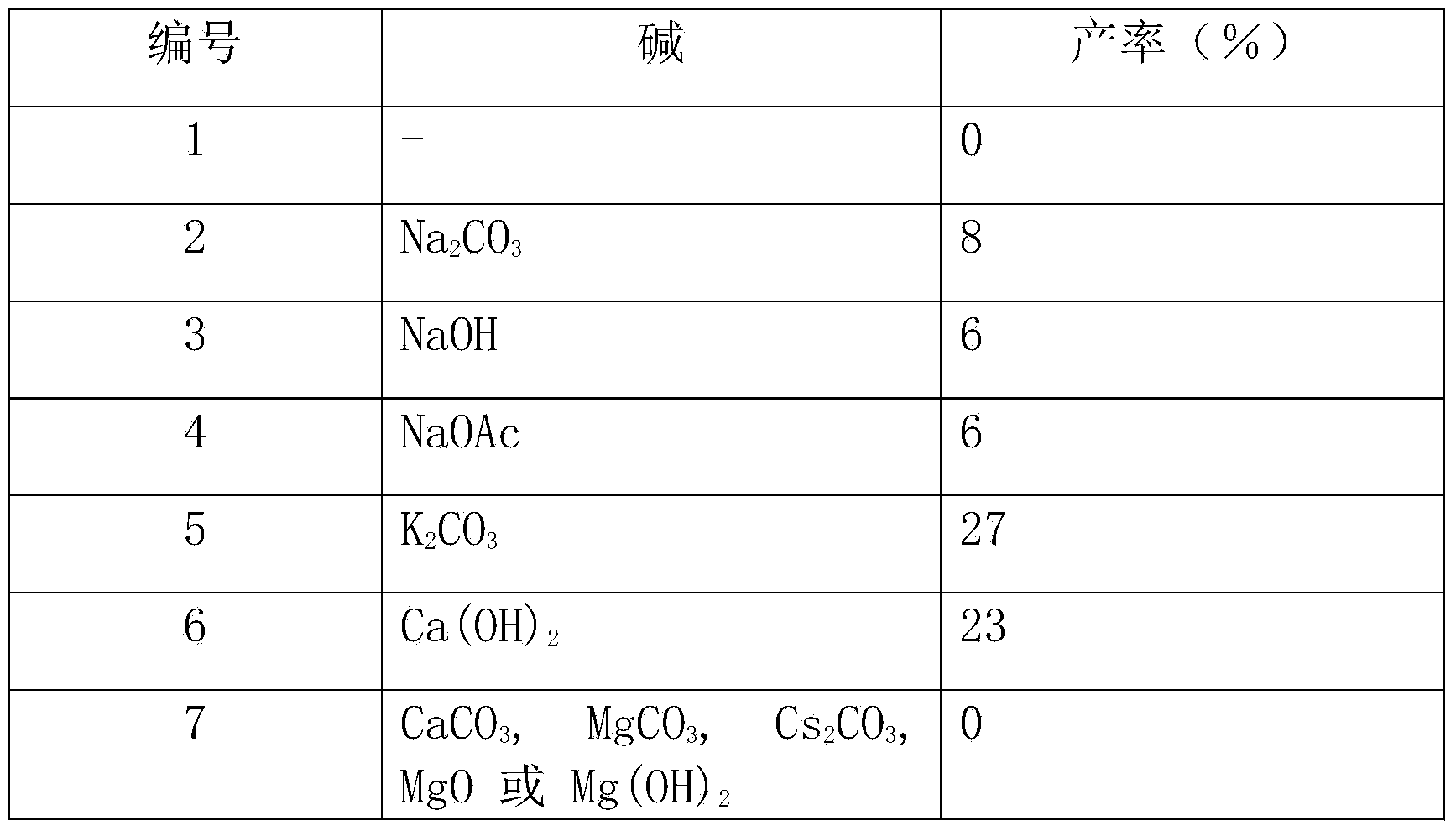
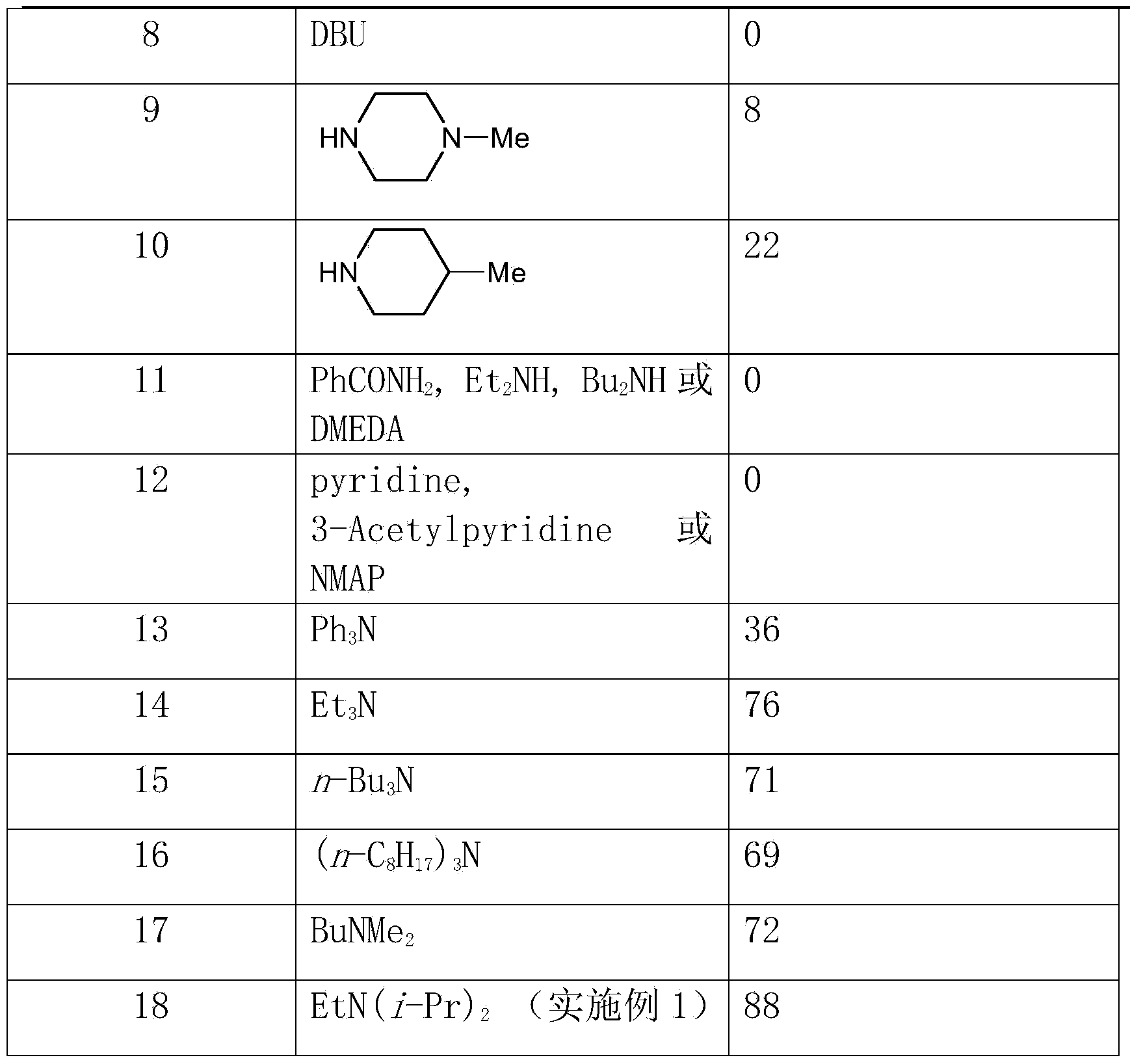
[0031] Other conditions are the same as in Example 1, and the reaction using different bases is checked, and the experimental results are shown in Table 1.
[0032] The inspection of different alkali effects in table 1
[0033]
[0034]
[0035] From the above results, it can be seen that the effect of using most organic or inorganic bases is very poor, but the effect of using tertiary amines is good. And the effect of using ethyl diisopropylamine will far surpass other tertiary amines (embodiment 1).
Embodiment 3
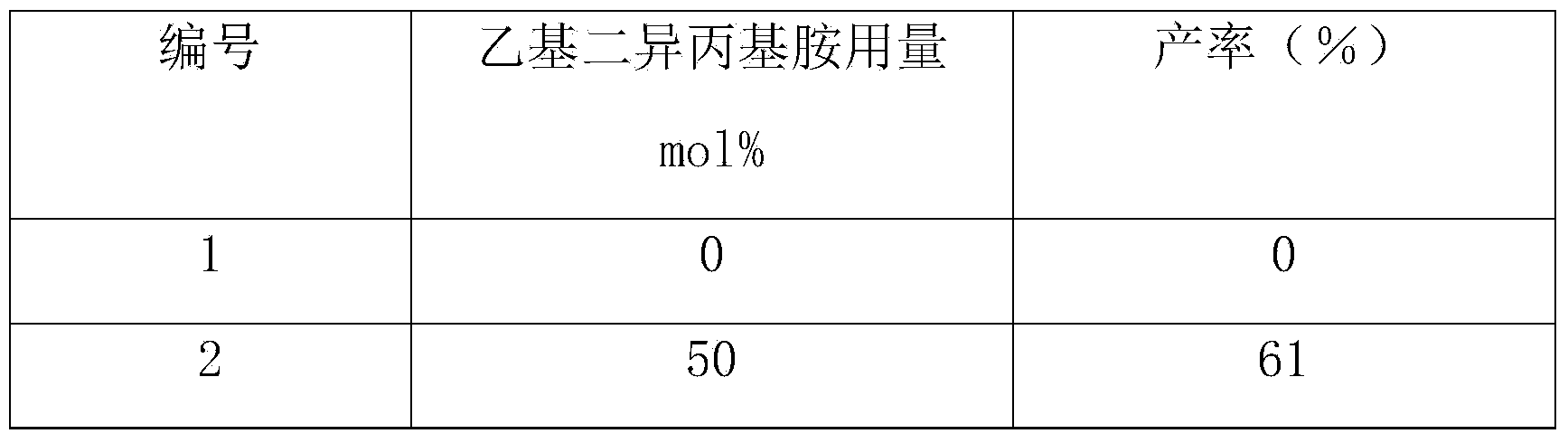
[0037] Other conditions are the same as in Example 1, and the reactions under different ethyldiisopropylamine dosages are checked, and the experimental results are shown in Table 2.
[0038] The inspection of different ethyldiisopropylamine consumption of table 2
[0039]
[0040]
[0041] From the above results, it can be known that the dosage of ethyldiisopropylamine is preferably 100 mol% (based on reactants) (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com