Stereoselective method for preparing ursodeoxycholic acid
A technology of ursodeoxycholic acid and stereoselectivity, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of immature electrochemical synthesis methods, achieve high-efficiency preparation, high conversion rate, and short preparation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
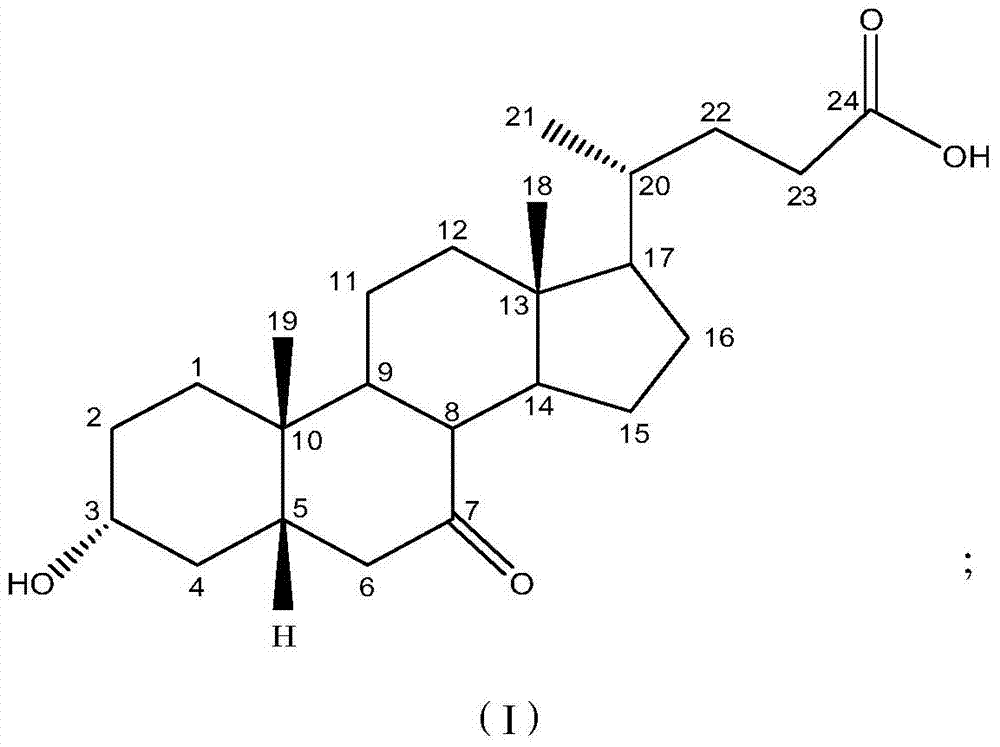
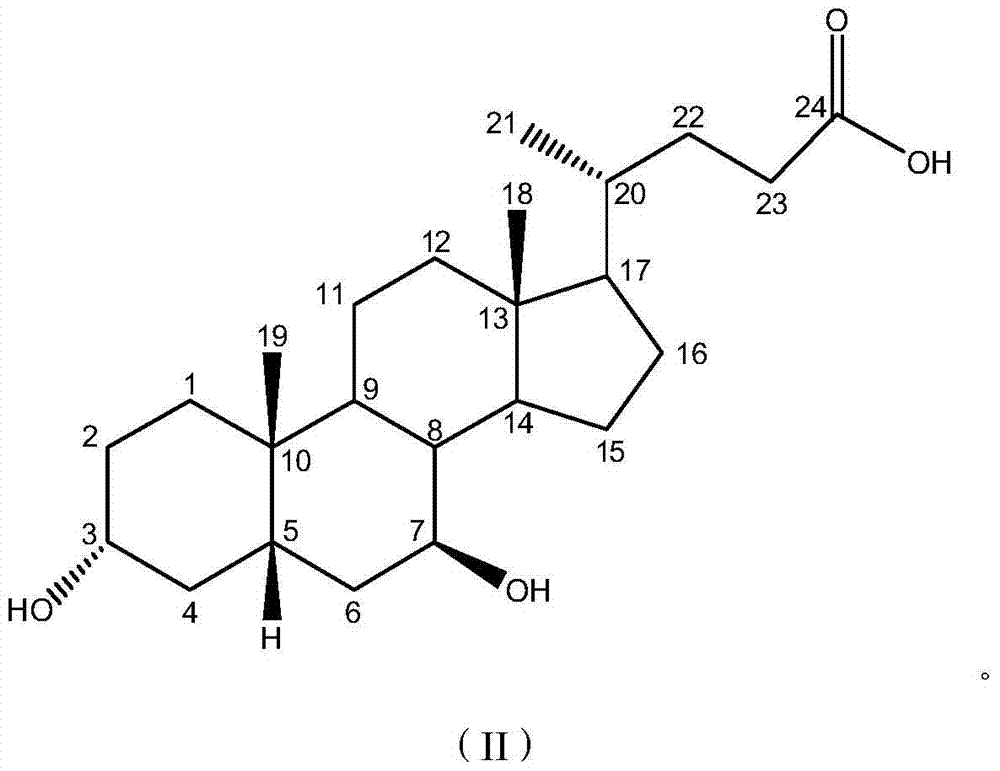
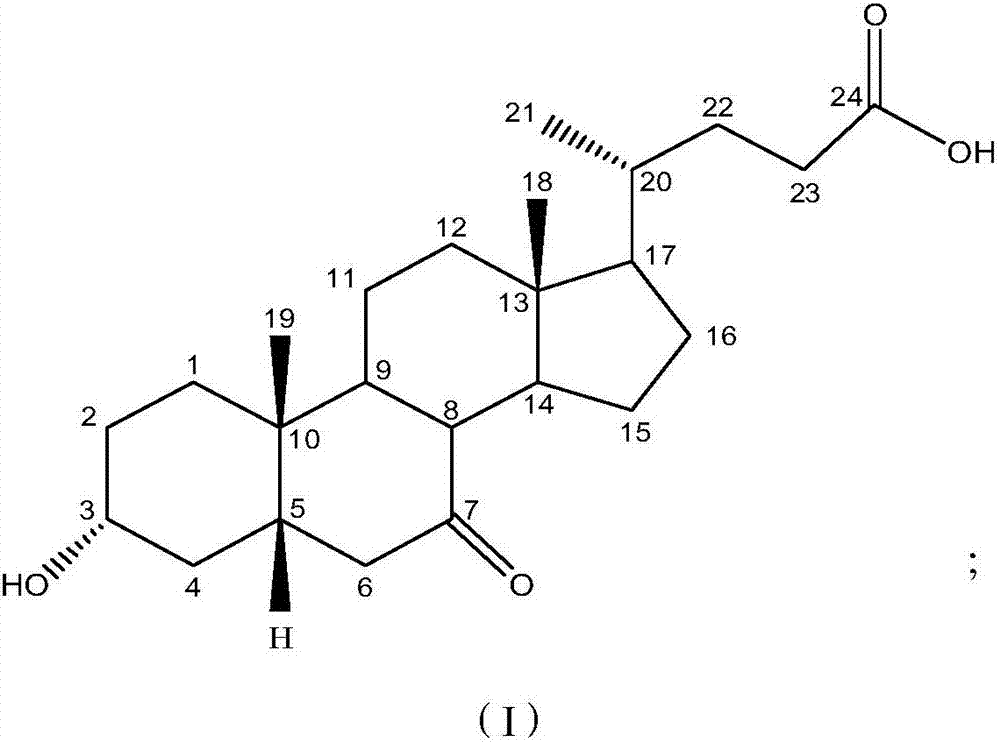
[0027] Dissolve 0.3g KBr in 12ml of water, 2.0g of 7-ketolithocholic acid in 110ml of methanol, and add 10ml of dimethylformamide, then mix the two solutions into the cathode cell as the catholyte; 10% dilute sulfuric acid is used as the anolyte; the lead electrode is used as the cathode, and the ruthenium-titanium electrode network is used as the anode; the HF101 strong-acid cation exchange membrane is used as the diaphragm, and the water bath is 50°C, and the constant current is electrolyzed, and the current density is 85A m -2 , Stop electrolysis when 7K-LCA disappears after thin layer chromatography. 1.932 g of UDCA crude product was obtained, the conversion rate by HPLC was 89.1%, and the purity was 52.3%.
Embodiment 2
[0029] Dissolve 0.3g KBr in 12ml water, dissolve 2.0g 7-ketolithocholic acid in 110ml methanol and add 5ml dimethyl sulfoxide, then mix the two solutions and put them into the cathode cell as catholyte; 5% dilute sulfuric acid is used as the anolyte; the lead electrode is used as the cathode, the ruthenium-titanium electrode network is used as the anode; the HF101 strong acid type cation exchange membrane is used as the diaphragm, the water bath is 65°C, and the constant current is electrolyzed, and the current density is 82A m -2 , Stop electrolysis when 7K-LCA disappears after thin layer chromatography. 1.894 g of crude UDCA was obtained, the conversion rate was 88.2% and the purity was 78.7% as measured by HPLC.
Embodiment 3
[0031] Dissolve 0.3g KBr in 12ml water, dissolve 2.5g 7-ketolithocholic acid in 110ml ethanol and add in 3ml dimethylacetamide, then mix the two solutions and put them into the cathode cell as catholyte; The 5% dilute sulfuric acid is used as the anolyte; the lead electrode is used as the cathode, and the PbO2 / Ti electrode mesh is used as the anode; the HF101 strong acid type cation exchange membrane is used as the diaphragm, and the water bath is 60°C, and the constant current is electrolyzed, and the current density is 85A m -2, Stop electrolysis when 7K-LCA disappears after thin layer chromatography. 1.914 g of crude UDCA was obtained, the conversion rate of the raw material detected by HPLC was 74.3%, and the purity was 55.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com