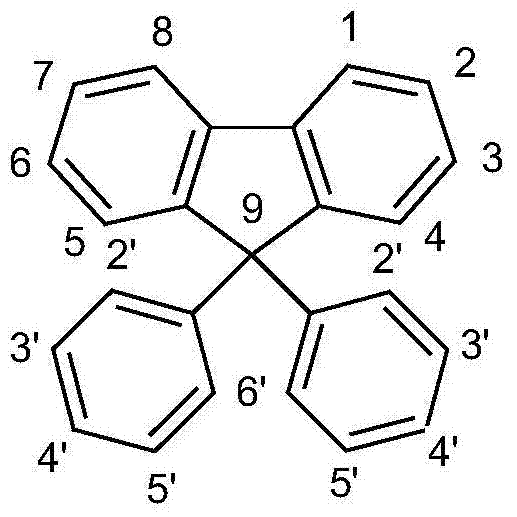
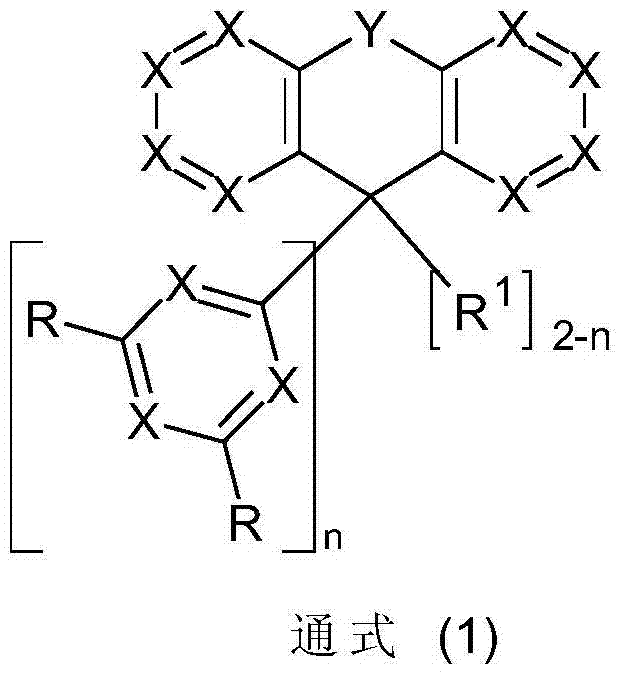
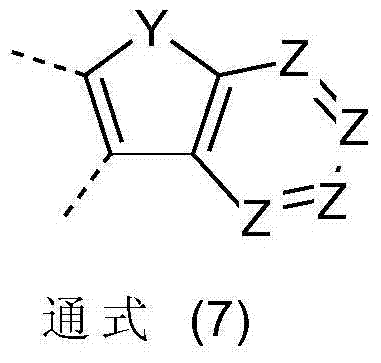
Fluorine derivatives for organic electroluminescence devices
A technology of compound and general formula, applied in the field of fluorine derivatives used in organic electroluminescent devices, can solve the problem that substituents do not give importance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Embodiment 1: Synthetic 9,9-bis(3,5-dibromophenyl) fluorene
[0149]
[0150] The corresponding Grignard compound was prepared from a mixture of 144.5 g (620 mmol) of 2-bromobiphenyl and 15.3 g (580 mmol) of magnesium in 500 ml of tetrahydrofuran and 250 ml of ethylene glycol dimethyl ether. A suspension of 224.0 g (450 mmol) of bis(3,5-dibromophenyl)ketone in 1000 ml tetrahydrofuran was then added at room temperature, and the mixture was stirred for a further twelve hours. The solvent was removed in vacuo, 1000 ml of glacial acetic acid and 5 ml of hydrogen bromide were added to the residue, and the mixture was stirred for one hour. The suspension was heated at reflux for half an hour and stirred at room temperature for 12 hours. The solid was filtered off with suction, washed three times with 300 ml of ethanol and recrystallized twice from toluene. Yield: 183.2g (289mmol), 64.3%, purity about 99.8% (high pressure liquid chromatography).
[0151] Analogously to E...
Embodiment 4
[0153] Embodiment 4: Synthetic 9,9-bis(3,5-diphenylphenyl) fluorene
[0154]
[0155]1.0g (3.3mmol) of tri-o-tolylphosphine and 0.5g (2.2mmol) of palladium (II) acetate were added to 30.4g (48mmol) of 9,9-bis(3,5-dibromophenyl) which was stirred well ) fluorene, 35.4g (290mmol) phenylboronic acid and 121.0g (570mmol) tripotassium phosphate in the suspension of the mixture of 300ml toluene, 300ml 1,4-dioxane and 300ml water, the mixture was then refluxed Heat for 3 hours. After cooling, the organic phase is separated off, washed three times with 150 ml of water each and filtered through silica gel. The solvent is removed in vacuo, the residue is dissolved in 200 ml of ethanol, filtered off with suction and washed three times with 100 ml of ethanol. The solid was recrystallized three times from chlorobenzene and, after drying, was sublimed twice in vacuo (p=1×10 -5 mbar, T=320°C). Yield: 11.1g (18mmol), 37.1%, purity about 99.9% (high pressure liquid chromatography).
[...
Embodiment 8
[0158] Embodiment 8: Synthetic 9,9-bis(3,5-diphenylaminophenyl) fluorene
[0159]
[0160] 101mg (0.50mmol) of tri-tert-butylphosphine and 56mg (0.25mmol) of palladium (II) acetate were added to 25.4g (40mmol) of 9,9-bis(3,5-dibromophenyl)fluorene which was stirred well , 33.8 g (200 mmol) of diphenylamine and 21.1 g (220 mmol) of [missing] in 500 ml of toluene were suspended, and the mixture was heated under reflux for 5 hours. After cooling, the solution was filtered through silica gel and evaporated to dryness in vacuo. The residue was stirred at 60° C. in 600 ml of a 1:1 mixture of ethanol and water for 1 hour, filtered off with suction, washed five times with 250 ml of ethanol and dried in vacuo. The beige solid was recrystallized five times from dimethylformamide and three times from chlorobenzene, dried in vacuo and sublimed twice (p=1×10 -5 mbar, T=350°C). Yield: 10.2g (10mmol), 25.0%, purity 99.9% (high pressure liquid chromatography), T g = 99.8°C.
[0161] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com