Copolymer and organic solar cell using same
A copolymer, non-replaced technology, applied in the direction of circuits, photovoltaic power generation, electrical components, etc., can solve the problems of cost increase, achieve the effect of improving open circuit voltage, small band gap, improving efficiency and service life characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] The copolymer can be produced based on the production examples described later.
[0157] Copolymers according to the present specification can be prepared using multi-step chemical reactions. After synthesizing monomers through alkylation reactions, Grignard reactions, Suzuki coupling reactions, and Stille coupling reactions, etc., final copolymers can be synthesized through carbon-carbon coupling reactions such as Stille coupling reactions. When the substituent to be introduced is a boronic acid or a borate compound, a Suzuki coupling reaction can be used; and when the substituent to be introduced is a tributyltin compound, a Stille coupling reaction can be used; however, the method of preparation is not limited here.
[0158] In addition, the specification provides an organic solar cell including a copolymer comprising the A unit, the B unit, and the C unit.
[0159] In one embodiment of the present specification, an organic solar cell includes: a first electrode; a...
Embodiment 1
[0186] Embodiment 1. monomer synthesis-1 (the synthesis of 2,5-bis(trimethylstannyl)thiophene)
[0187]
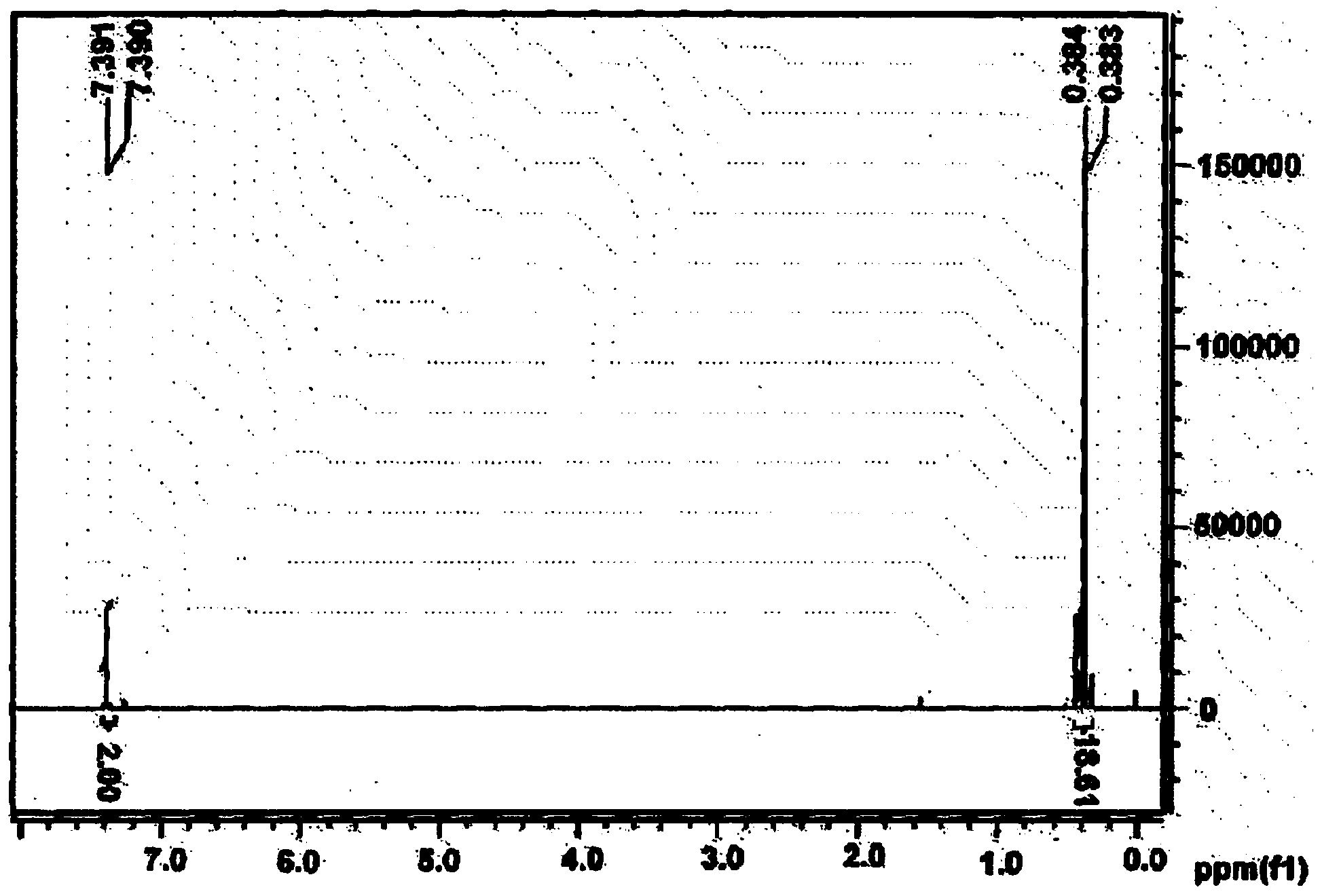
[0188] After 2,5-dibromothiophene (9.68 g, 40.0 mmol) was placed in tetrahydrofuran (200 ml) and dissolved, the temperature was lowered to -78°C. At this temperature, 1.6M n-butyllithium (n-BuLi) dissolved in hexane (55 ml, 88 mmol) was slowly added thereto, and the mixture was stirred for 1 hour. Immediately thereafter, 1 M trimethyltin chloride dissolved in THF (100 ml, 100 mmol) was added thereto, the temperature was raised to room temperature, and the mixture was stirred for 12 hours. The solution was poured into ice, extracted 3 times with diethyl ether, washed 3 times with water, and treated with magnesium sulfate (MgSO 4 ) to remove residual water. After removing the remaining solvent under vacuum, the solution was recrystallized from methanol to give a white solid.
[0189] Yield: 73.1%
[0190] figure 1 is a schematic diagram showing the NMR spectrum of th...
Embodiment 2
[0191] Example 2. Monomer Synthesis-2 (Synthesis of 2,5-bis-trimethylstannyl-thieno[3,2-b]thiophene)
[0192]
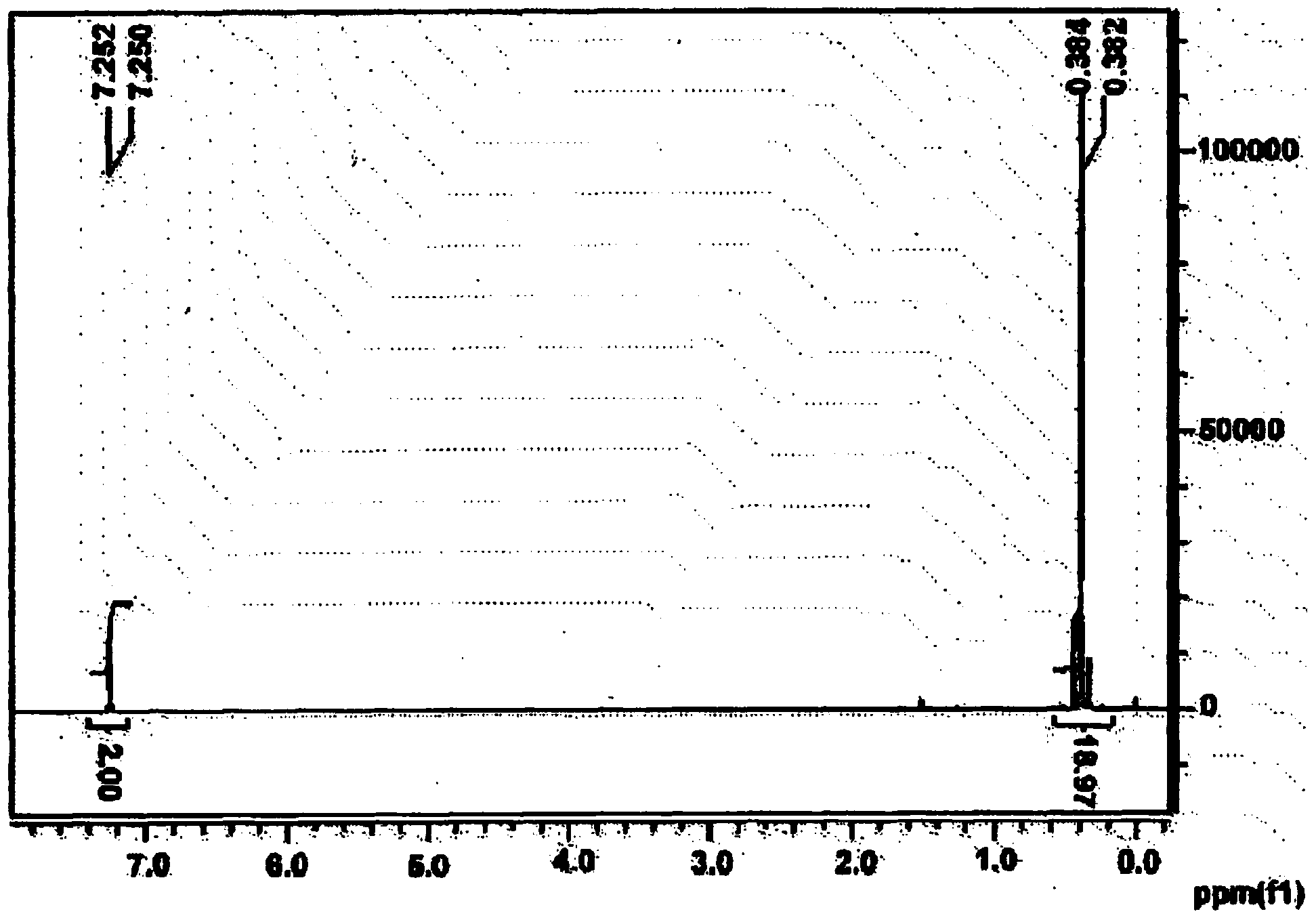
[0193] After thieno[3,2-b]thiophene (1.5 g, 10.7 mmol) was placed in tetrahydrofuran (50 ml) and dissolved, the temperature was lowered to -78°C. At this temperature, 1.6M n-butyllithium (n-BuLi) dissolved in hexane (14.7 ml, 23.53 mmol) was slowly added thereto, and the mixture was stirred for 30 minutes. Thereafter, the temperature was raised to 0°C, the mixture was stirred at this temperature for 1 hour, and the temperature was lowered to -78°C again. 1M trimethyltin chloride dissolved in THF (26.7ml, 26.7mmol) was added thereto at once, the temperature was raised to room temperature, and the mixture was stirred for 12 hours. The solution was poured into ice, extracted 3 times with diethyl ether, washed 3 times with water, and treated with magnesium sulfate (MgSO 4 ) to remove residual water. After removing the remaining solvent under vacuum, the solution wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com