A degradable multi-branched glycopeptide hydrogel against postoperative scars and its preparation method
A multi-branched, hydrogel technology, applied in the field of biomedicine, can solve the problems of increasing patient pain, high price, and difficulty in artificial synthesis of proteoglycans, so as to reduce production and use costs, reduce dosage, and have good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
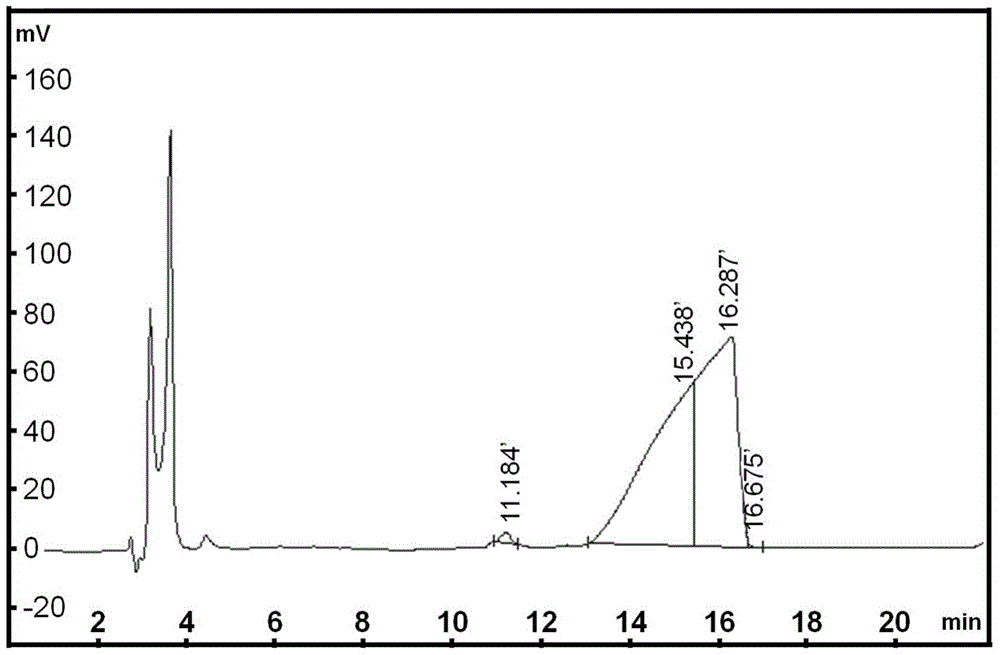
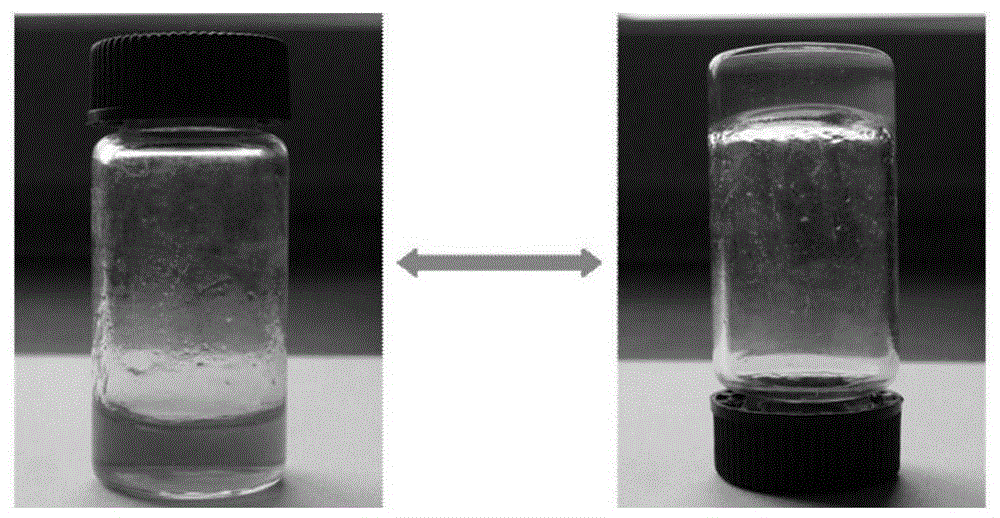
Image
Examples
Embodiment 1
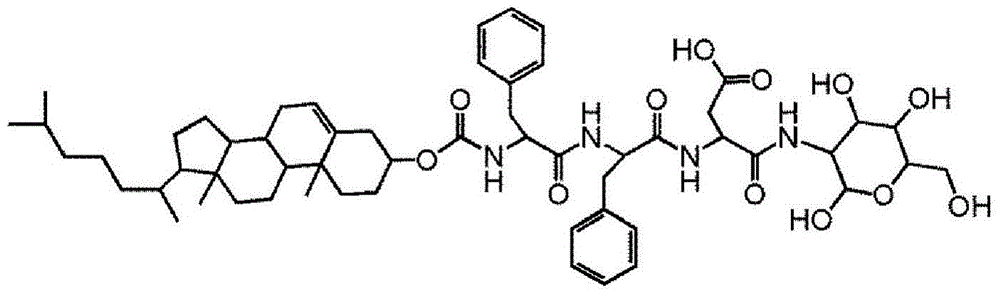
[0031] (1) Preparation of tripeptide Chol-Phe-Phe-Asp(OtBu)-OH modified with hydrophobic functional group cholesterol (cholesterol, Chol) containing good biocompatibility
[0032]Using 2-chloro-trityl chloride resin (the effective chlorine substitution degree of the resin is 1.08mmol / g) as the solid phase carrier, the cholesterol-linked short peptide Chol-Phe-Phe-Asp was prepared using a peptide solid-phase automatic synthesizer (OtBu)-OH. The peptide chain extends from the carbon end to the nitrogen end on the resin. The specific preparation steps are as follows: Weigh 2.0g of 2-chloro-trityl chloride resin (the amount of total available chlorine is 2.0g × 1.08mmol / g=2.16mmol, then soak the resin with 15mL DMF for 1 hour. Remove the DMF , 15 mL DMF solution of FMOC-Asp(OtBu)-OH (2 × 2.16mmol) and diisopropylethylamine (DIEA, 6 × 2.16mmol) was added to the resin, and the reaction was stirred at room temperature for 1.5 hours. Remove the reaction liquid, wash the resin three ...
Embodiment 2
[0044] (1) Preparation of tripeptide Chol-Phe-Phe-Asp-OH modified with hydrophobic cholesterol (cholesterol, Chol) containing good biocompatibility
[0045]Using 2-chloro-trityl chloride resin (the effective chlorine substitution degree of the resin is 1.08mmol / g) as the solid phase carrier, the cholesterol-linked short peptide Chol-Phe-Phe-Asp was prepared using a peptide solid-phase automatic synthesizer (OtBu)-OH. The peptide chain extends from the carbon end to the nitrogen end on the resin. The specific preparation steps are as follows: Weigh 2.0g of 2-chloro-trityl chloride resin (the amount of total available chlorine is 2.0g × 1.08mmol / g=2.16mmol, then soak the resin with 15mL DMF for 1 hour. Remove the DMF , 15 mL DMF solution of FMOC-Asp(OtBu)-OH (2 × 2.16mmol) and diisopropylethylamine (DIEA, 6 × 2.16mmol) was added to the resin, and the reaction was stirred at room temperature for 1.5 hours. Remove the reaction liquid, wash the resin three times with 15 mL DMF, t...
Embodiment 3
[0052] (1) Preparation of tetrapeptide Chol-Phe-Phe-Asp-Asp-OH modified with good biocompatibility and hydrophobic cholesterol (cholesterol, Chol)
[0053] Using 2-chloro-trityl chloride resin (the effective chlorine substitution degree of the resin is 1.08mmol / g) as the solid phase carrier, the cholesterol-linked short peptide Chol-Phe-Phe-Asp was prepared using a peptide solid-phase automatic synthesizer (OtBu)-OH. The peptide chain extends from the carbon end to the nitrogen end on the resin. The specific preparation steps are as follows: Weigh 2.0g of 2-chloro-trityl chloride resin (the amount of total available chlorine is 2.0g × 1.08mmol / g=2.16mmol, then soak the resin with 15mL DMF for 1 hour. Remove the DMF , 15 mL DMF solution of FMOC-Asp(OtBu)-OH (2 × 2.16mmol) and diisopropylethylamine (DIEA, 6 × 2.16mmol) was added to the resin, and the reaction was stirred at room temperature for 1.5 hours. Remove the reaction liquid, wash the resin three times with 15 mL DMF, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com