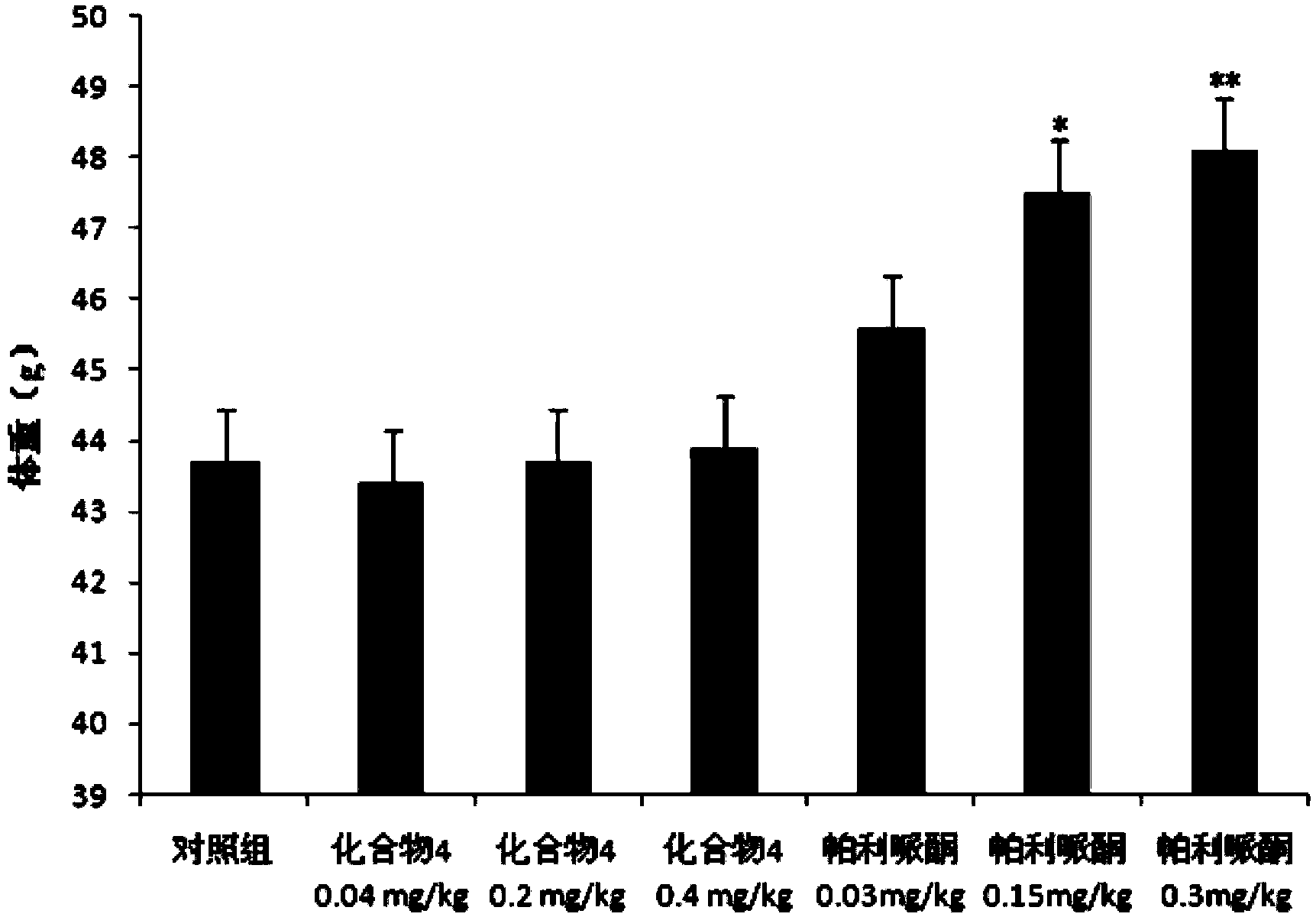
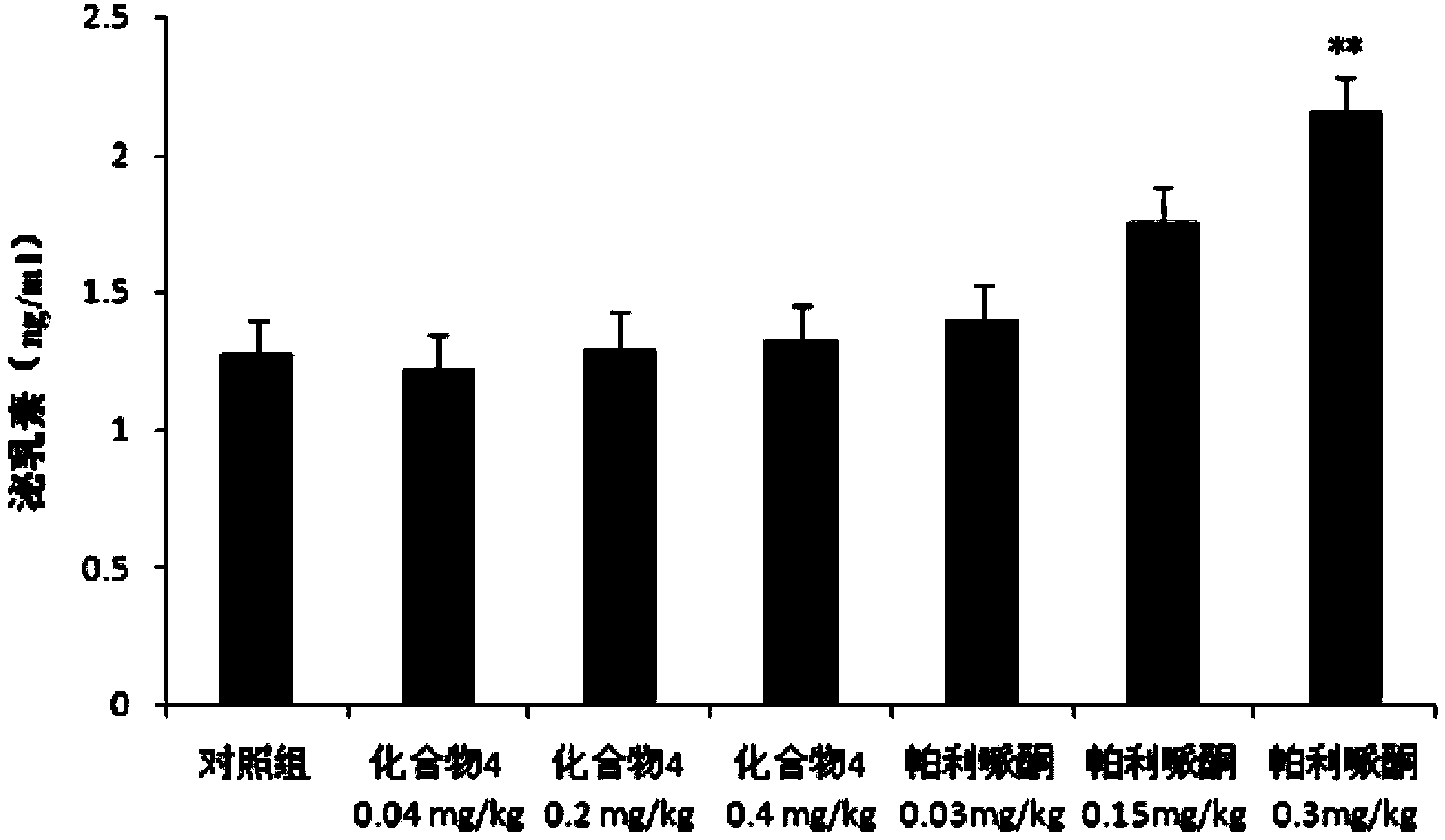
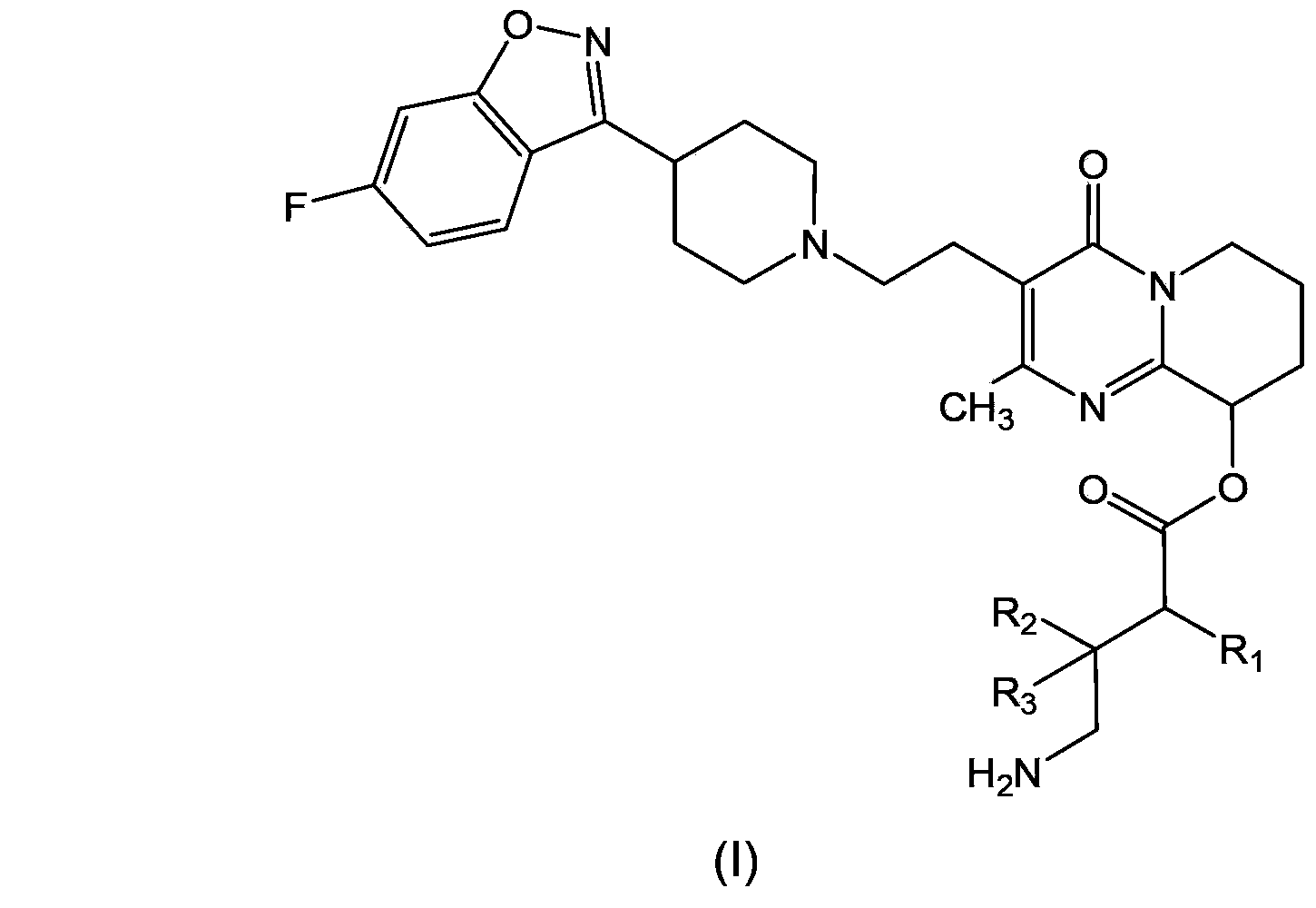
Paliperidone amino acid derivatives and application thereof
An alkyl and methyl technology, applied in the field of paliperidone amino acid derivatives and its application, can solve problems such as weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1, 3-(2-(4-(6-fluoro-benzisoxazole)-3-piperidinyl)-ethyl)-2-methyl-4-oxygen-6,7,8, 9‐tetrahydro‐4H‐pyrido[1,2‐a]9‐pyrimidinyl‐4‐aminobutyrate hydrochloride
[0057] (1) Take 4-aminobutyric acid (10.3g, 100mmol) and add it to the reaction flask, add 50ml water, and 100ml acetone, add triethylamine (110mmol), stir at room temperature until completely dissolved, add Boc in batches Acid anhydride (26.2g, 120mmol), reacted at room temperature for 4h. Stop the reaction, evaporate acetone under reduced pressure, extract with ether 50ml×2, discard several layers, adjust the pH of the aqueous layer to 4‐5 with 10% dilute hydrochloric acid, extract with ethyl acetate 200ml×3, combine several layers , dried with anhydrous magnesium sulfate, filtered, and the filtrate was spin-dried to obtain 18.5 g of a colorless oil, with a yield of 91.1%. MS(ESI)m / z204.2([M+H] + ).
[0058] (2) Take 2.0g (10mmol) of the first step product, add 50ml of dichloromethane, add 3.4g (9mmol...
Embodiment 2
[0060] Example 2, 3-(2-(4-(6-fluoro-benzisoxazole)-3-piperidinyl)-ethyl)-2-methyl-4-oxygen-6,7,8, 9‐tetrahydro‐4H‐pyrido[1,2‐a]9‐pyrimidinyl‐3‐(aminomethyl)‐5‐methyl‐hexanoate hydrochloride
[0061] Using 3-aminomethyl-5-methylhexanoic acid instead of 4-aminobutyric acid, the target compound 2 was prepared according to the method of Example 1.
[0062] The structural formula of the target compound 2 is shown in Table 1, and its melting point is 185-187°C. 1 H NMR(DMSO)δ11.15(s,1H),8.19‐8.23(m,1H),7.69‐7.73(m,1H),7.30‐7.35(m,1H),5.68(t,J=8Hz,2H ),3.95‐3.98(m,1H),3.71‐3.76(m,3H),3.45‐3.51(m,4H),3.11‐3.18(m,4H),2.92‐2.96(m,1H),2.51‐2.56 (m,4H),2.51(s,2H),2.38‐2.40(m,2H),2.31(s,3H),2.23‐2.27(m,2H),1.72‐1.99(m,7H),1.02(d , J=6.6Hz, 6H). MS(ESI)m / z568.3([M+H] + ).
Embodiment 3
[0063] Example 3, 3-(2-(4-(6-fluoro-benzoisoxazole)-3-piperidinyl)-ethyl)-2-methyl-4-oxygen-6,7,8, 9‐tetrahydro‐4H‐pyrido[1,2‐a]9‐pyrimidinyl‐4‐amino‐2‐methylbutyrate hydrochloride
[0064] Using 4-amino-2-methylbutyric acid instead of 4-aminobutyric acid, the target compound 3 was prepared according to the method of Example 1.
[0065] The structural formula of the target compound 3 is shown in Table 1, and its melting point is 188-189°C. 1H NMR(DMSO)δ11.20(s,1H),8.17‐8.20(m,1H),7.70‐7.76(m,1H),7.29‐7.33(m,1H),5.70(t,J=8Hz,2H ),3.89‐3.92(m,1H),3.72‐3.77(m,3H),3.45‐3.51(m,4H),3.14‐3.18(m,4H),2.95‐2.99(m,1H),2.51‐2.57 (m,4H),2.38‐2.41(m,2H),2.99(s,3H),2.23‐2.27(m,3H),1.92‐1.99(m,5H),1.43(d,J=6Hz,2H) . MS(ESI)m / z526.3([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com