Pharmaceutical preparation containing imatinib mesylate and preparation method thereof
A technology for imatinib mesylate and pharmaceutical preparations, applied in the field of pharmaceutical preparations, can solve the problems of unsuitable α crystal form and difficult medicinal use of imatinib mesylate, and achieves reduced types of excipients and high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation of embodiment 1~3 different wetting agent tablet
[0031] The composition of table 1 tablet (1000 pieces)
[0032] Element
Example 1
Example 2
Example 3
Imatinib mesylate
120g
120
120
55g
55
55
Crospovidone
40g
40
40
silica
3g
3g
3g
2g
2g
2g
Opadry
6.6g
6.6g
6.6g
50% ethanol
75% ethanol
[0033] 1.1 Preparation
[0034] Mix the pretreated imatinib mesylate and internal pharmaceutical excipients in a wet granulation pot, then granulate with a wetting agent, then dry and granulate, add external excipients and mix, then compress into tablets, package Clothes.
[0035] 1.2 Evaluation
[0036] Method: Chinese Pharmacopoeia 2010 Edition II XC Second Method Paddle Method
[0037] Medium: 900ml of hydrochloric acid solution with pH 1....
Embodiment 8-10
[0059] Adopt the preparation method of embodiment 1-3, wherein wetting agent is 75% ethanol
[0060] Element
Example 8
Example 9
Example 10
Imatinib mesylate
120g
120g
120g
60.3g
49.5g
35.3g
Crospovidone
33g
44g
55g
silica
4.5g
3.3g
7.5g
1.1g
2.2g
1.1g
Opadry
1.1g
1.1g
1.1g
75% ethanol
75% ethanol
75% ethanol
[0061] Gained preparation dissolution rate result is as follows:
[0062] Table 5 Dissolution evaluation results
[0063]
[0064] Result: the dissolution results of the above examples show that the dissolution effects of the preparation examples of the present invention are stable and have good reproducibility.
experiment example 1
[0065] Experimental example 1, stability test
[0066] In order to verify whether the preparation containing the α crystal form in the present invention will transform into the β crystal form in a long-term storage environment, the inventor prepared 3 batches of samples using the prescription and preparation process of Example 2 and Example 9, respectively named For samples 2-1, 2-2, 2-3 and 9-1, 9-2, 9-3, samples were taken to detect the transformation of the preparation crystal form in different environments.
[0067] Table 6 Crystal Form Stability Monitoring (25±2°C, RH=60%±15%)
[0068]
[0069]
[0070] Table 7 Crystal Form Stability Monitoring (45±2°C, RH=75%±5%)
[0071]
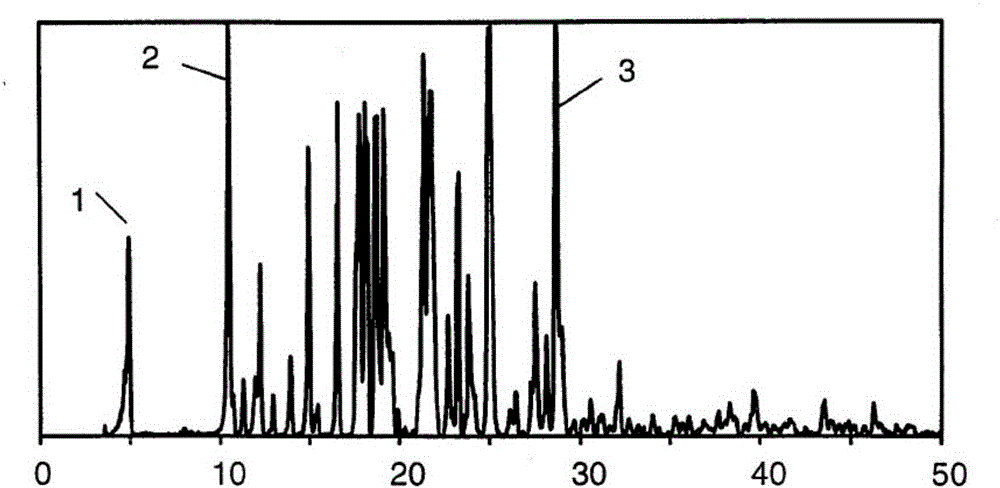
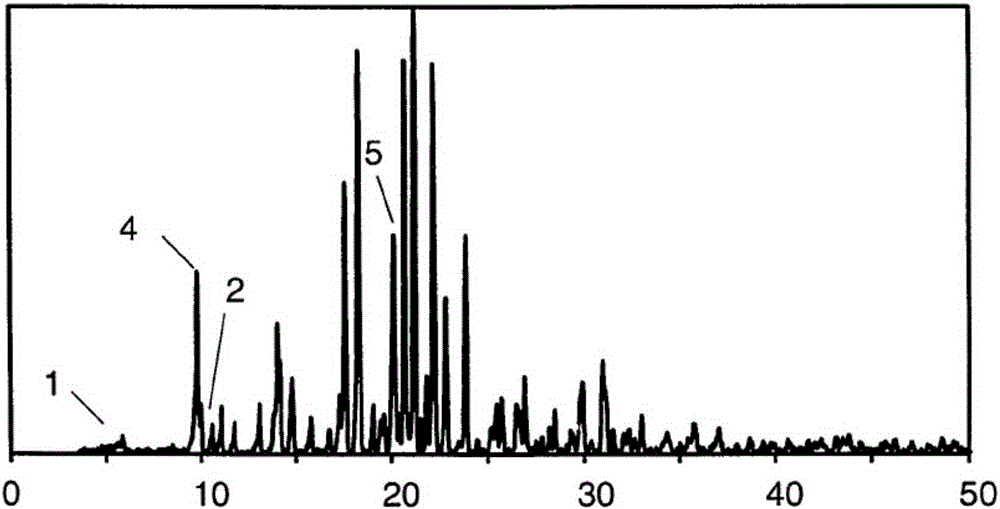
[0072] Note: The criterion for distinguishing whether the crystal is transformed is that at least one diffraction peak deviates from the normal error by 0.2°. If the marker peak (diffraction peak with 2θ of 4.9) is missing, it can be determined that the α crystal form has undergone transform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com