Method for recovering sliver from silver-bearing waste liquor
A waste liquid recovery and waste liquid technology, applied in the field of metallurgical industry, can solve the problems of incomplete separation of Ag and impurities, high energy consumption of concentrated crystallization method, and narrow application scope, etc. Simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
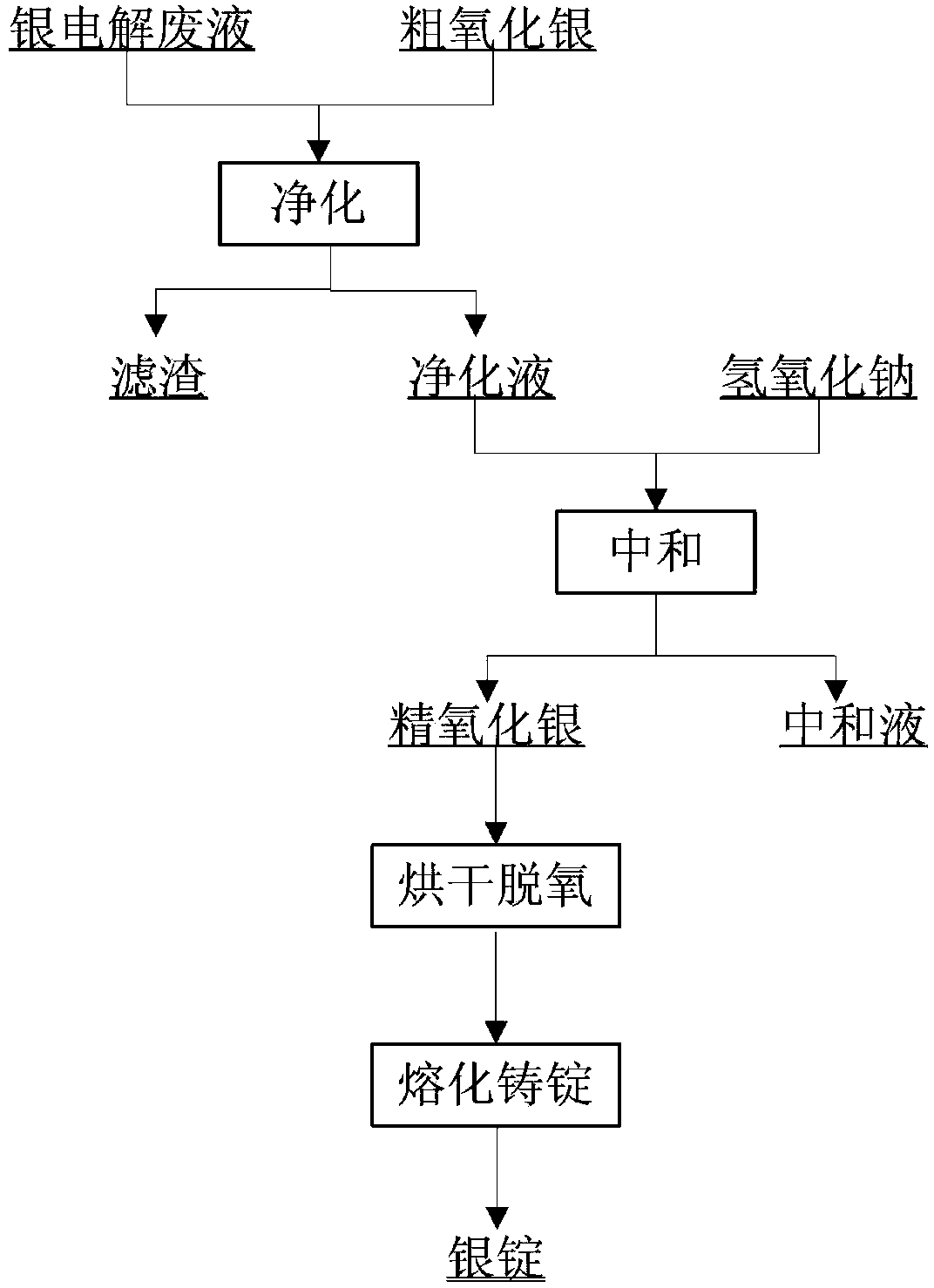
[0022] 1m the silver-containing waste liquid with a silver ion concentration of 103.15g / L 3 Pump into the purification kettle, start stirring, slowly add NaOH until the end pH of the solution reaches 10-11 to prepare crude silver oxide (Ag: 67.23%; Cu: 6.51%) for later use. Another 1m of silver-containing waste liquid 3 Pump into the purification kettle, turn on the stirring, slowly add the prepared crude silver oxide, when the pH of the solution is measured to be 6.4, turn on the steam, heat it to 80°C and keep it for 1 hour, press filter to obtain 870L of purified liquid, pump it to the precipitation kettle, the test results: Ag + :135.42g / L, Cu 2+ :1.12mg / L, Pb 2+ :3.41mg / L, Bi 3+ : Low; desilver residue is piled up for later use. Turn on the precipitation tank and stir, slowly add industrial-grade NaOH to the end of the solution pH value of 10-11, press filter to obtain a neutralization solution (Ag + : 1.78mg / L) efflux, and refined silver oxide (Ag: 92.97%). After drying t...
Embodiment 2
[0024] 1m the silver-containing waste liquid with a silver ion concentration of 84.48g / L 3 Pump into the purification kettle, start stirring, and slowly add NaOH until the end pH of the solution reaches 10-11 to prepare crude silver oxide (Ag: 63.48%; Cu: 7.15%) for later use. In addition, pump 850L of silver-containing waste liquid into the purification kettle, start stirring, slowly add coarse silver oxide, and when the pH of the solution is measured to be 6.8, turn on the steam, heat it to 80℃ and keep it for 1.5h, press filter to obtain 760L of purified liquid, and pump to Precipitation kettle, test result: Ag + :130.56g / L, Cu 2+ :1.42mg / L, Pb 2+ :3.08mg / L, Bi 3+ :0.22mg / L; the silver removal residue is piled up for later use. Turn on the precipitation tank and stir, slowly add industrial-grade NaOH until the end pH of the solution is 10-11, press filter to obtain the neutralization solution (Ag: 1.78 mg / L) and drain off and refined silver oxide (Ag: 93.01%). After the refi...
Embodiment 3
[0026] 1m the silver-containing waste liquid with a silver ion concentration of 110.14g / L 3 Pump into the purification kettle, start stirring, and slowly add NaOH until the end pH of the solution reaches 10-11 to prepare crude silver oxide (Ag: 68.74%; Cu: 6.43%) for later use. Another 1m of silver-containing waste liquid 3 Pump into the purification kettle, turn on the stirring, slowly add the prepared crude silver oxide, when the pH of the solution is 6.4, turn on the steam, heat it to 80°C and keep it for 1 hour, press filter to obtain 800L of purified liquid, pump it to the precipitation kettle, the test results: Ag + :156.17g / L, Cu 2+ :2.16mg / L, Pb 2+ :4.54mg / L, Bi 3+ : Low; desilver residue is piled up for later use. Turn on the precipitation tank and stir, slowly add industrial-grade NaOH to the end of the solution pH value of 10-11, press filter to obtain a neutralization solution (Ag + : 0.87mg / L) efflux, and refined silver oxide (Ag: 92.94%). After the refined silver ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com